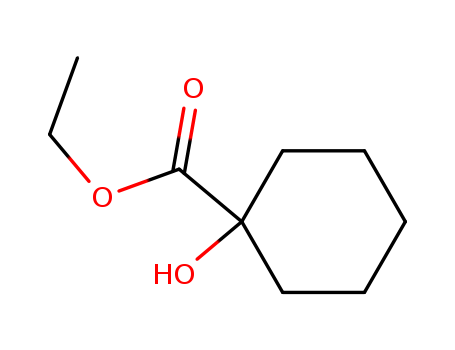
1127-01-1
- Product Name:1-Hydroxy-1-Cyclohexylmethanecarboxylate
- Molecular Formula:C9H16O3
- Purity:99%
- Molecular Weight:172.224
Product Details;
CasNo: 1127-01-1
Molecular Formula: C9H16O3
Manufacturer supply good quality 1-Hydroxy-1-Cyclohexylmethanecarboxylate 1127-01-1 with stock
- Molecular Formula:C9H16O3
- Molecular Weight:172.224
- Vapor Pressure:0.0147mmHg at 25°C
- Refractive Index:1.4532
- Boiling Point:227.909 °C at 760 mmHg
- PKA:13.28±0.20(Predicted)
- Flash Point:86.187 °C
- PSA:46.53000
- Density:1.104 g/cm3
- LogP:1.24470
ETHYL 1-HYDROXYCYCLOHEXANE-CARBOXYLATE(Cas 1127-01-1) Usage
|
General Description |
Ethyl 1-Hydroxycyclohexane Carboxylate is a chemical compound with the molecular formula C9H16O3. This organic compound belongs to the family of cyclohexanecarboxylic acids and derivatives. Its systematic name is Ethyl (1-hydroxycyclohexyl)acetate. It exists as a colorless liquid at room temperature, and is typically utilized in a variety of chemical reactions due to its reactive nature. It is not widely produced or used, and details about its risk or safety profiles are not easily available, suggesting it is generally applied in more specialized or limited-scale industrial chemistry settings. |
InChI:InChI=1/C9H16O3/c1-2-12-8(10)9(11)6-4-3-5-7-9/h11H,2-7H2,1H3
1127-01-1 Relevant articles
Production process of high-purity and high-yield spirodiclofen
-
Paragraph 0047; 0057; 0067, (2019/03/08)
The invention relates to the technical f...
Preparation method of spirodiclofen and intermediate of spirodiclofen
-
Paragraph 0006; 0011; 0035-0037, (2018/12/13)
The invention discloses a preparation me...
Spirodiclofen and spiromesifen - Novel acaricidal and insecticidal tetronic acid derivatives with a new mode of action
Bretschneider, Thomas,Benet-Buchholz, Jordi,Fischer, Reiner,Nauen, Ralf
, p. 697 - 701 (2007/10/03)
The broad spectrum acaricides spirodiclo...
Cyclic Ether Formation in Superacid Media
Carr, Graham,Whittaker, David
, p. 359 - 366 (2007/10/02)
The formation of ethers in superacids by...
1127-01-1 Process route
-
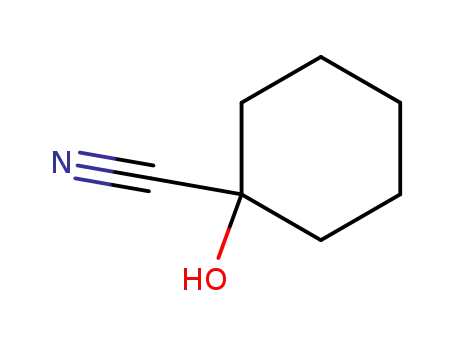
- 931-97-5
1-hydroxy-1-cyclohexanecarbonitrile

-
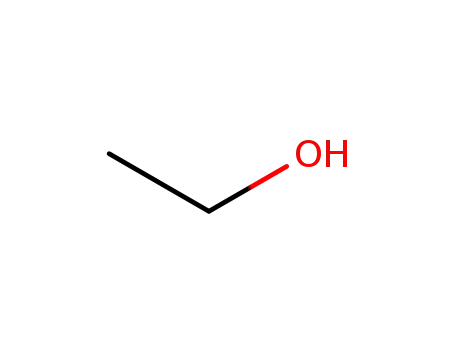
- 64-17-5
ethanol

-
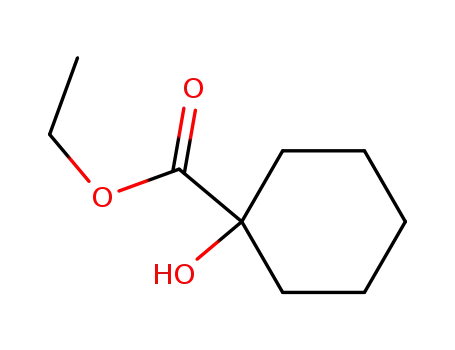
- 1127-01-1
ethyl 1-hydroxycyclohexanecarboxylate
| Conditions | Yield |
|---|---|
|
1-hydroxy-1-cyclohexanecarbonitrile; ethanol; With hydrogenchloride; at 0 - 25 ℃; for 14h;
With water; at 20 ℃;
|
88.6% |
|
With hydrogenchloride; In water; for 14h; Solvent; Concentration; Cooling with ice;
|
-
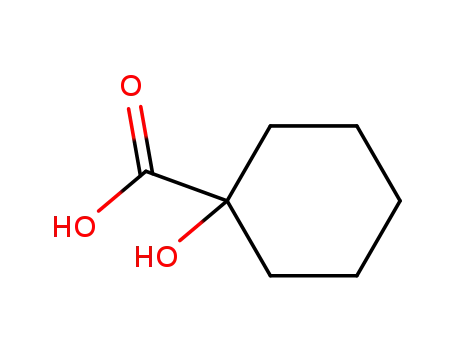
- 1123-28-0
1-hydroxy-cyclohexanecarboxylic acid

-

- 64-17-5
ethanol

-

- 1127-01-1
ethyl 1-hydroxycyclohexanecarboxylate
| Conditions | Yield |
|---|---|
|
With sulfuric acid; In benzene; Heating;
|
42% |
|
With sulfuric acid;
|
|
|
With hydrogenchloride;
|
|
|
With toluene-4-sulfonic acid;
|
1127-01-1 Upstream products
-
1123-28-0

1-hydroxy-cyclohexanecarboxylic acid
-
64-17-5

ethanol
-
931-97-5

1-hydroxy-1-cyclohexanecarbonitrile
-
3289-28-9
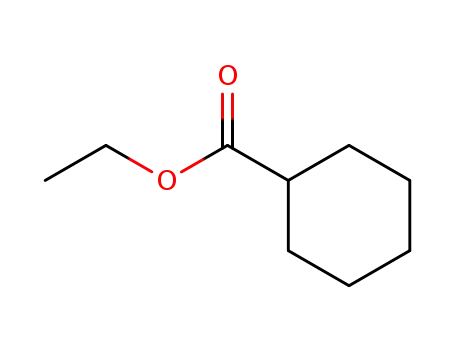
ethyl cyclohexanecarboxylate
1127-01-1 Downstream products
-
57865-45-9
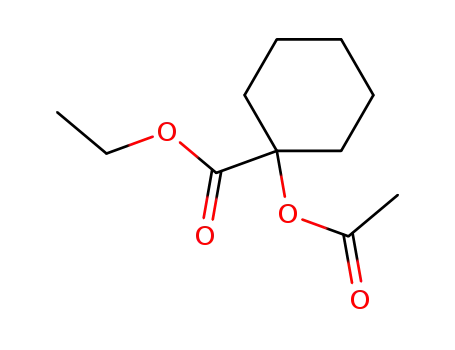
1-acetoxy-cyclohexanecarboxylic acid ethyl ester
-
7759-32-2
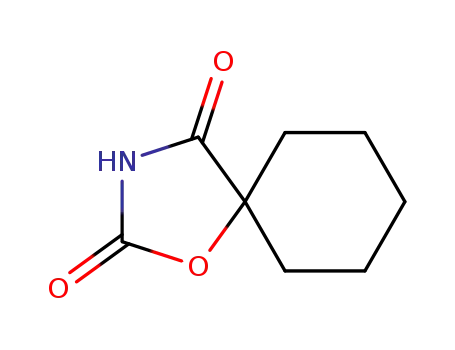
5,5-Pentamethylenoxazolidin-2,4-dion
-
1617-22-7
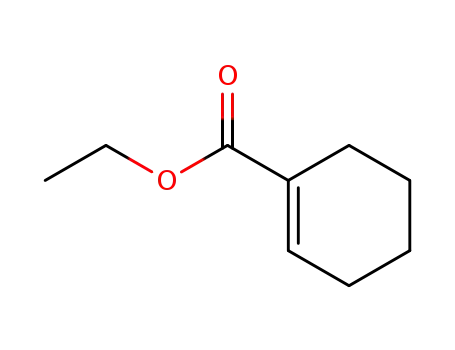
cyclohex-1-enecarboxylic acid ethyl ester
-
636-82-8
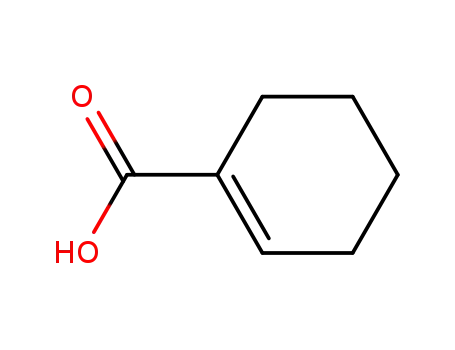
cyclohexene-1-carboxylic acid
Relevant Products
-
5-(2′-Hydroxy-3′-Naphthamide)-2-Benzimidazolone
CAS:26848-40-8
-
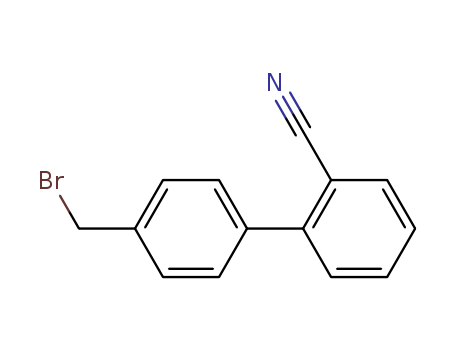
2'-Cyano-4-Bromomethyl Biphenyl
CAS:114772-54-2
-
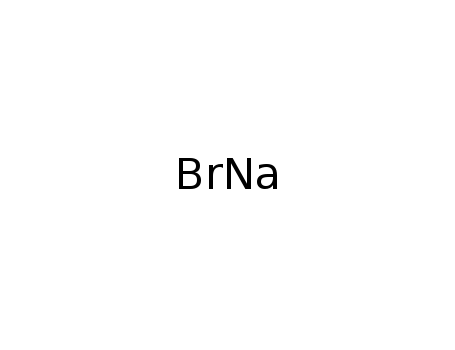
Sodium Bromide
CAS:7647-15-6