112-92-5
- Product Name:Stearyl Alcohol
- Molecular Formula:C18H38O
- Purity:99%
- Molecular Weight:270.499
Product Details;
CasNo: 112-92-5
Molecular Formula: C18H38O
Appearance: White flakes
Top Quality Stearyl Alcohol 112-92-5 Hot Sell In Stock
- Molecular Formula:C18H38O
- Molecular Weight:270.499
- Appearance/Colour:White flakes
- Vapor Pressure:<0.01 mm Hg ( 38 °C)
- Melting Point:55-58 °C
- Refractive Index:1.45
- Boiling Point:334.3 °C at 760 mmHg
- PKA:15.20±0.10(Predicted)
- Flash Point:138.7 °C
- PSA:20.23000
- Density:0.837 g/cm3
- LogP:6.24020
1-Hydroxyoctadecane(Cas 112-92-5) Usage
|
Production Methods |
Historically, stearyl alcohol was prepared from sperm whale oil but is now largely prepared synthetically by reduction of ethyl stearate with lithium aluminum hydride. |
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 49, p. 2438, 1984 DOI: 10.1021/jo00187a028Synthetic Communications, 12, p. 463, 1982 DOI: 10.1080/00397918208065953 |
|
Health Hazard |
Mildly toxic by ingestion. Questionable carcinogen with experimental neoplastigenic data. A skin and eye irritant. |
|
Fire Hazard |
Flammable when exposed to heat or flame; can react with oxidizing materials. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes. |
|
Flammability and Explosibility |
Notclassified |
|
Pharmaceutical Applications |
1-Octadecanol is used in cosmetics and topical pharmaceutical creams and ointments as a stiffening agent. By increasing the viscosity of an emulsion, stearyl alcohol increases its stability. 1-Octadecanol also has some emollient and weak emulsifying properties, and is used to increase the water-holding capacity of ointments, e.g. petrolatum. In addition, 1-Octadecanol has been used in controlled-release tablets, suppositories, and microspheres.It has also been investigated for use as a transdermal penetration enhancer. |
|
Safety |
Stearyl alcohol is generally considered to be an innocuous, nontoxic material. However, adverse reactions to stearyl alcohol present in topical preparations have been reported. These include contact urticaria and hypersensitivity reactions, which are possibly due to impurities contained in stearyl alcohol rather than stearyl alcohol itself. The probable lethal oral human dose is greater than 15 g/kg. LD50 (rat, oral): 20 g/kg |
|
storage |
Stearyl alcohol is stable to acids and alkalis and does not usually become rancid. It should be stored in a well-closed container in a cool, dry place. |
|
Purification Methods |
Crystallise octadecanol from MeOH, or dry Et2O and *C6H6, then fractionally distil it in vacuo. Also purify it by column chromatography. Free it from cetyl alcohol by zone refining. [Beilstein 1 IV 1888.] |
|
Incompatibilities |
Incompatible with strong oxidizing agents and strong acids. |
|
Regulatory Status |
Included in the FDA Inactive Ingredients Database (oral tablets, rectal topical, and vaginal preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients. |
|
Definition |
ChEBI: Octadecan-1-ol is a long-chain primary fatty alcohol consisting of a hydroxy function at C-1 of an unbranched saturated chain of 18 carbon atoms. It has a role as a plant metabolite, a human metabolite and an algal metabolite. It is a long-chain primary fatty alcohol, a fatty alcohol 18:0 and a primary alcohol. It derives from a hydride of an octadecane. |
|
Application |
1-Octadecanol is a long chain primary alcohol that is used in the production of emulsions, textile oils, antifoam agents, and lubricants. Other large scale applications include the manufacture of alkyl amines, tertiary amines, ethoxylates, halides/mercaptans, and polymerization stabilizers. It generally occurs as a mixture of solid alcohols whose primary constituent is 1-octadecanol. It occurs naturally in sperm whale oil and has been isolated from the hyperthermophilic bacterium Pyrococcus furiosus.1-Octadecanol has been used to model the plant epicuticular wax layer for an investigation by differential scanning calorimetry and Fourier transform infrared spectroscopy.The use of 1-octadecanol to prepare microsphere formulations for such compounds as paclitaxel and indomethacin has been described. |
|
General Description |
Mixed monolayers of 1-octadecanol and ethylene glycol monooctadecyl ether were studied to investigate their evaporation suppressing performance. The rate dependence of the collapse pressure for an octadecanolmonolayer using axisymmetric drop shape analysis has been investigated. |
InChI:InChI=1/C18H38O/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19/h19H,2-18H2,1H3
112-92-5 Relevant articles
Selective Hydrodeoxygenation of Vegetable Oils and Waste Cooking Oils to Green Diesel Using a Silica-Supported Ir–ReOx Bimetallic Catalyst
Liu, Sibao,Simonetti, Trent,Zheng, Weiqing,Saha, Basudeb
, p. 1446 - 1454 (2018)
High yields of diesel-range alkanes are ...
An unconventional DCOx favored Co/N-C catalyst for efficient conversion of fatty acids and esters to liquid alkanes
Li, Jiang,Liu, Jiaxing,Zhang, Junjie,Wan, Tong,Huang, Lei,Wang, Xintian,Pan, Runze,An, Zhidong,Vlachos, Dionisios G.
, (2020)
Cobalt (Co) catalysis has recently attra...
Permeable composite membrane as a catalytically active contactor for hydrogenation reactions
Minyukova, T. P.,Shtertser, N. V.,Khassin, A. A.,Yurieva, T. M.
, p. 107 - 110,4 (2012)
The efficiency of using of the permeable...
Highly Selective Hydrodecarbonylation of Oleic Acid into n-Heptadecane over a Supported Nickel/Zinc Oxide-Alumina Catalyst
Li, Guangci,Zhang, Feng,Chen, Lei,Zhang, Chuanhui,Huang, He,Li, Xuebing
, p. 2646 - 2653 (2015)
The production of second-generation biod...
Influence of the operating conditions and kinetic analysis of the selective hydrogenation of oleic acid on Ru-Sn-B/Al2O3 catalysts
Sánchez, María A.,Pouilloux, Yannick,Mazzieri, Vanina A.,Pieck, Carlos L.
, p. 552 - 558 (2013)
The influence of the operating condition...
Synergistic Interaction between Oxides of Copper and Iron for Production of Fatty Alcohols from Fatty Acids
Kandel, Kapil,Chaudhary, Umesh,Nelson, Nicholas C.,Slowing, Igor I.
, p. 6719 - 6723 (2015)
The selective hydrogenation of fatty aci...
Kinetics of hydrodeoxygenation of stearic acid using supported nickel catalysts: Effects of supports
Kumar, Pankaj,Yenumala, Sudhakara Reddy,Maity, Sunil K.,Shee, Debaprasad
, p. 28 - 38 (2014)
The hydrodeoxygenation of fatty acids de...
The selective hydrogenation of ethyl stearate to stearyl alcohol over Cu/Fe bimetallic catalysts
He, Limin,Li, Xiaoru,Lin, Weiwei,Li, Wei,Cheng, Haiyang,Yu, Yancun,Fujita, Shin-Ichiro,Arai, Masahiko,Zhao, Fengyu
, p. 143 - 149 (2014)
Bimetallic and monometallic catalysts in...
An efficient hydrogenation catalytic model hosted in a stable hyper-crosslinked porous-organic-polymer: From fatty acid to bio-based alkane diesel synthesis
Sarkar, Chitra,Shit, Subhash Chandra,Dao, Duy Quang,Lee, Jihyeon,Tran, Ngoc Han,Singuru, Ramana,An, Kwangjin,Nguyen, Dang Nam,Le, Quyet Van,Amaniampong, Prince Nana,Drif, Asmaa,Jerome, Francois,Huyen, Pham Thanh,Phan, Thi To Nga,Vo, Dai-Viet N.,Thanh Binh, Nguyen,Trinh, Quang Thang,Sherburne, Matthew P.,Mondal, John
, p. 2049 - 2068 (2020)
In this study, a Pd-based catalytic mode...
Spectroscopy and Reactivity of Dialkoxy Acenes
Brega, Valentina,Kanari, Sare Nur,Doherty, Connor T.,Che, Dante,Sharber, Seth A.,Thomas, Samuel W.
, p. 10400 - 10407 (2019)
Photochemical oxidation of acenes can be...
Impact of the oxygen defects and the hydrogen concentration on the surface of tetragonal and monoclinic ZrO2 on the reduction rates of stearic acid on Ni/ZrO2
Foraita, Sebastian,Fulton, John L.,Chase, Zizwe A.,Vjunov, Aleksei,Xu, Pinghong,Barth, Eszter,Camaioni, Donald M.,Zhao, Chen,Lercher, Johannes A.
, p. 2423 - 2434 (2015)
The role of the specific physicochemical...
Supported iron nanoparticles for the hydrodeoxygenation of microalgal oil to green diesel
Kandel, Kapil,Anderegg, James W.,Nelson, Nicholas C.,Chaudhary, Umesh,Slowing, Igor I.
, p. 142 - 148 (2014)
Iron nanoparticles supported on mesoporo...
In situ hydrogenation and decarboxylation of oleic acid into heptadecane over a Cu-Ni alloy catalyst using methanol as a hydrogen carrier
Zhang, Zihao,Yang, Qiwei,Chen, Hao,Chen, Kequan,Lu, Xiuyang,Ouyang, Pingkai,Fu, Jie,Chen, Jingguang G.
, p. 197 - 206 (2018)
In this work, supported Cu-Ni bimetallic...
Controlling Hydrodeoxygenation of Stearic Acid to n-Heptadecane and n-Octadecane by Adjusting the Chemical Properties of Ni/SiO2–ZrO2 Catalyst
Foraita, Sebastian,Liu, Yue,Haller, Gary L.,Baráth, Eszter,Zhao, Chen,Lercher, Johannes A.
, p. 195 - 203 (2017)
A series of SiO2–ZrO2 mixed oxides with ...
New bioactive sulfated metabolites from the Mediterranean tunicate Sidnyum turbinatum
Aiello,Carbonelli,Fattorusso,Iuvone,Menna
, p. 219 - 221 (2001)
In addition to the known sodium 3,7,11,1...
Selective hydrogenation of fatty acids to alcohols over highly dispersed ReOx/TiO2 catalyst the paper is dedicated to the living memory of Dr. Haldor Topsoe.
Rozmyslowicz, Bartosz,Kirilin, Alexey,Aho, Atte,Manyar, Haresh,Hardacre, Christopher,W?rn?, Johan,Salmi, Tapio,Murzin, Dmitry Yu.
, p. 197 - 207 (2015)
Production of fatty alcohols through sel...
Catalytic conversion of Jatropha oil to alkanes under mild conditions with a Ru/La(OH)3 catalyst
Guo, Jian-Hua,Xu, Guang-Yue,Shen, Fei,Fu, Yao,Zhang, Ying,Guo, Qing-Xiang
, p. 2888 - 2895 (2015)
The long-chain alkanes obtained from the...
Catalytic production of 1-octadecanol from octadecanoic acid by hydrotreating in a plug flow reactor
Potts, Thomas M.,Durant, Keiron,Hestekin, Jamie,Beitle, Robert,Ackerson, Michael
, p. 1643 - 1650,8 (2014)
1-Octadecanol (stearic alcohol) has uses...
Effective conversion of heteroatomic model compounds in microalgae-based bio-oils to hydrocarbons over β-Mo2C/CNTs catalyst
Liang, Junmei,Ding, Ranran,Wu, Yulong,Chen, Yu,Wu, Kejing,Meng, Yongqiang,Yang, Mingde,Wang, Yaowu
, p. 95 - 102 (2016)
Hydrotreatment of heteroatomic model com...
Hydrodeoxygenation of Palmitic and Stearic Acids on Phosphide Catalysts Obtained In Situ in Reaction Medium
Golubeva,Maksimov
, p. 1326 - 1330 (2019)
Abstract: Unsupported phosphide catalyst...
Balancing the efficacy vs. the toxicity of promiscuous natural products: Paclitaxel-based acid-labile lipophilic prodrugs as promising chemotherapeutics
Chittiboyina, Amar G.,Claudio, Pier Paolo,Haider, Saqlain,McChesney, James D.,Penfornis, Patrice
, (2021/10/19)
TumorSelect is an anticancer technology ...
MOF-derived hcp-Co nanoparticles encapsulated in ultrathin graphene for carboxylic acids hydrogenation to alcohols
Dong, Mei,Fan, Weibin,Gao, Xiaoqing,Zhu, Shanhui
, p. 201 - 211 (2021/06/03)
Highly efficient conversion of carboxyli...
Selective upgrading of biomass-derived benzylic ketones by (formic acid)–Pd/HPC–NH2 system with high efficiency under ambient conditions
Chen, Yuzhuo,Chen, Zhirong,Gong, Yutong,Mao, Shanjun,Ning, Honghui,Wang, Yong,Wang, Zhenzhen
, p. 3069 - 3084 (2021/11/16)
Upgrading biomass-derived phenolic compo...
Redox-active ligand based Mn(i)-catalyst for hydrosilylative ester reduction
Chakraborty, Soumi,Das, Arpan,Mandal, Swadhin K.
supporting information, p. 12671 - 12674 (2021/12/04)
Herein a Mn(i) catalyst bearing a redox-...
112-92-5 Process route
-
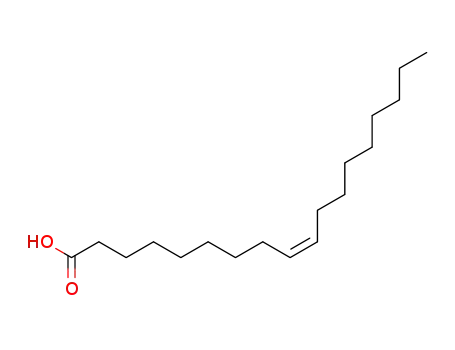
- 112-80-1,2027-47-6
cis-Octadecenoic acid

-

- 112-92-5
1-octadecanol

-
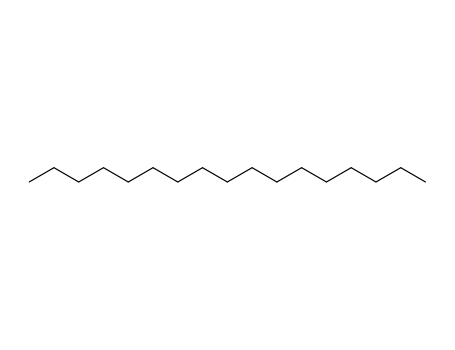
- 629-78-7
hepatdecane

-
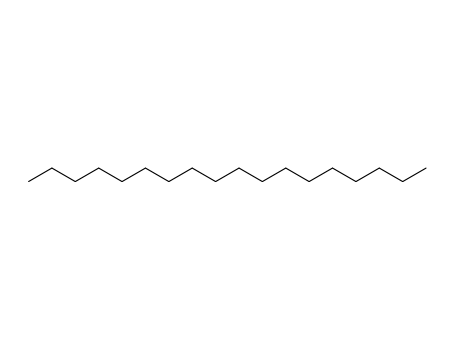
- 593-45-3
octadecane

-
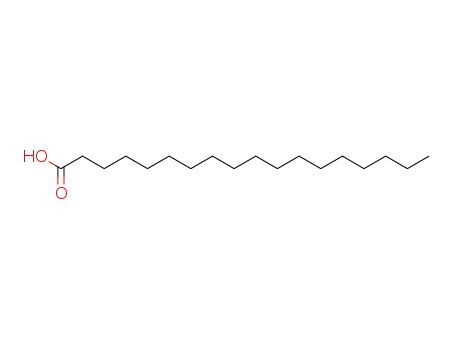
- 57-11-4
stearic acid
| Conditions | Yield |
|---|---|
|
With hydrogen; In hexane; at 290 ℃; under 22502.3 Torr; Temperature;
|
-
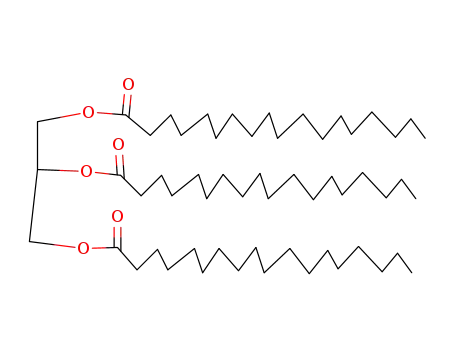
- 555-43-1
glycerol tristearate

-

- 112-92-5
1-octadecanol

-

- 629-78-7
hepatdecane

-

- 593-45-3
octadecane

-

- 638-66-4
n-Octadecanal

-
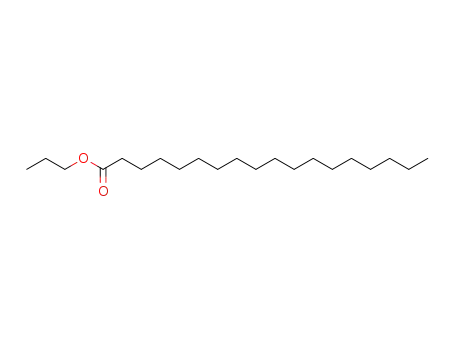
- 3634-92-2
propyl stearate

-
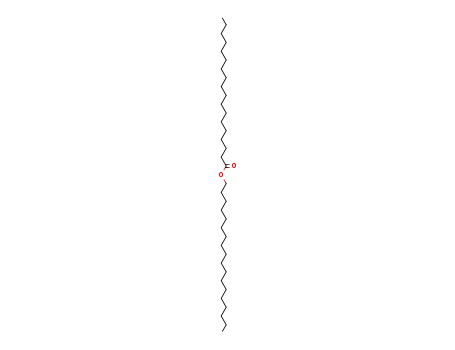
- 2778-96-3
octadecanoic acid, octadecyl ester

-
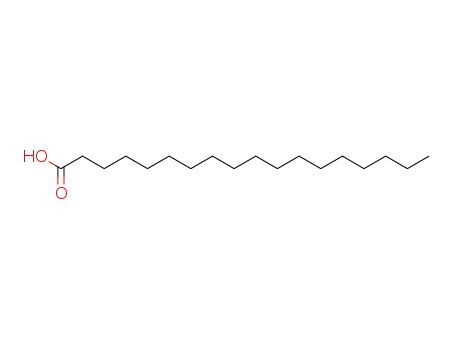
- 57-11-4
stearic acid
| Conditions | Yield |
|---|---|
|
With hydrogen; at 340 ℃; for 3h; under 15001.5 Torr;
|
112-92-5 Upstream products
-
50-00-0
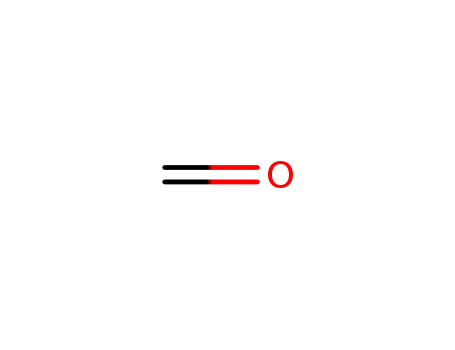
formaldehyd
-
143-28-2
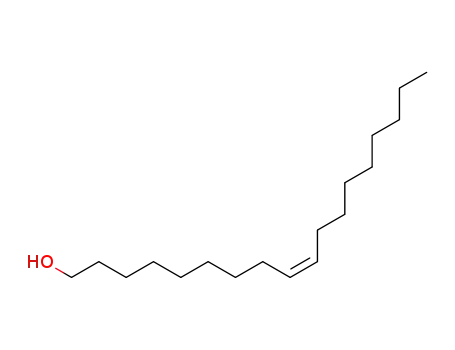
oleoyl alcohol
-
112-80-1

cis-Octadecenoic acid
-
111-62-6
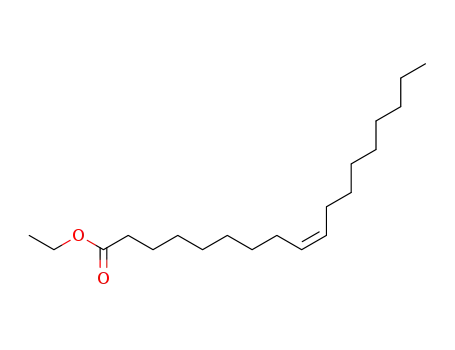
oleic acid ethyl ester
112-92-5 Downstream products
-
3234-84-2

octadecyl laurate
-
17181-26-9
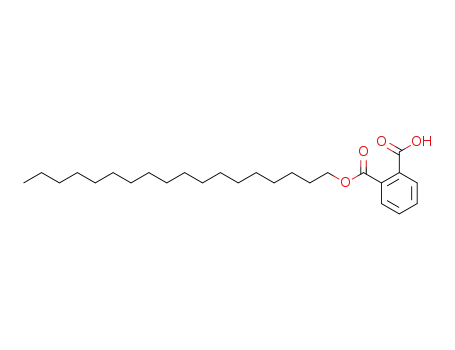
mono-octadecyl ortho-phthalate
-
18748-98-6
1-octadecanol, trimethylsilyl ether
-
3072-03-5
Sebacinsaeure-dioctadecylester
Relevant Products
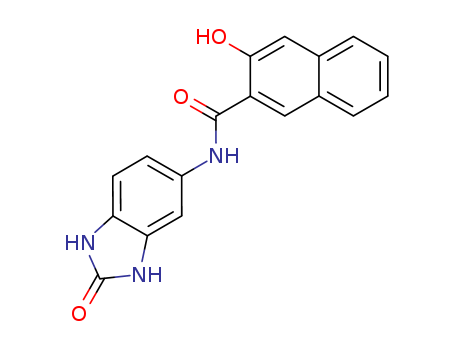
-
5-(2′-Hydroxy-3′-Naphthamide)-2-Benzimidazolone
CAS:26848-40-8
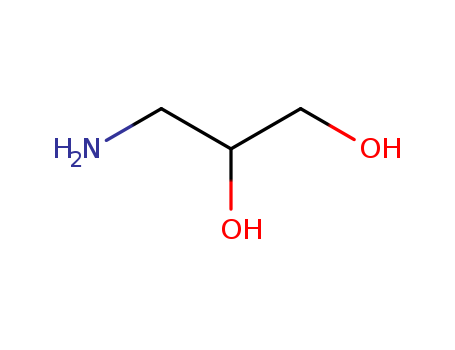
-
3-Amino-1,2-propanediol
CAS:616-30-8
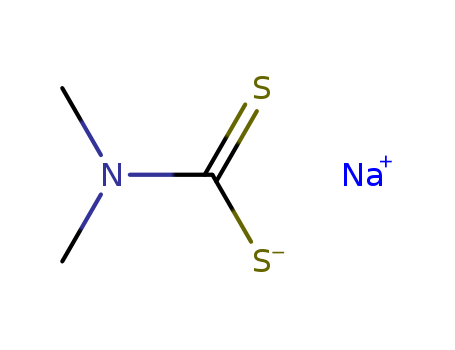
-
Sodium dimethyldithiocarbamate
CAS:128-04-1