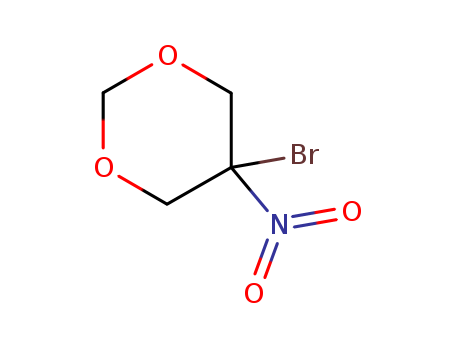
52-51-7
- Product Name:2-Bromo-2-nitro-1,3-propanediol
- Molecular Formula:C3 H6BrNO4
- Purity:99%
- Molecular Weight:199.989
Product Details;
CasNo: 52-51-7
Molecular Formula: C3 H6BrNO4
Appearance: off-white crystalline powder
Buy Quality 2-Bromo-2-nitro-1,3-propanediol 52-51-7 In Stock with Immediately Delivery
- Molecular Formula:C3 H6BrNO4
- Molecular Weight:199.989
- Appearance/Colour:off-white crystalline powder
- Vapor Pressure:1.45E-06mmHg at 25°C
- Melting Point:130- 133°C
- Refractive Index:1.574
- Boiling Point:358 °C at 760 mmHg
- PKA:12.02±0.10(Predicted)
- Flash Point:170.3 °C
- PSA:86.28000
- Density:2.018 g/cm3
- LogP:-0.13790
2-Bromo-2-nitro-1,3-propanediol(Cas 52-51-7) Usage
|
Production Methods |
Bronopol is synthesized by the reaction of nitromethane with paraformaldehyde in an alkaline environment, followed by bromination. After crystallization, bronopol powder may be milled to produce a powder of the required fineness. |
|
Manufacturing Process |
A mixture of 441 g (3 mols) of calcium chloride dihydrate, 61 g (1 mol) of nitromethane, 163 g (2 mols) of formalin (37% formaldehyde solution) and 470 ml of water was cooled to 0°C and mixed with 5 g of calcium hydroxide while stirring. The temperature thereby rose to 30°C. As soon as the temperature had fallen again, a further 32 g of calcium hydroxide (total of 0.5 mol) were added. The mixture was then cooled to 0°C and with intensive cooling and stirring, 159.8 g (1 mol, 51 ml) of bromine were dropped in at a rate so that the temperature remained at around 0°C. After the addition was ended, the mixture was stirred for a further 2 hours, when the reaction product separated in crystalline form. The product was quickly filtered on a suction filter and the crystalline sludge obtained was taken up in 450 ml of ethylene chloride and dissolved at reflux. Then by addition of magnesium sulfate, undissolved inorganic salts were separated and the solution was slowly cooled whereby 140 g (70% yield) of 2-bromo-2-nitropropane-1,3-diol precipitated in colorless crystals melting at 123°-124°C. |
|
Therapeutic Function |
Antiseptic |
|
Biological Functions |
Bronopol, 2-bromo-2-nitropropan-1,3-diol, is an aliphatic halogenonitro compound with potent antibacterial activity but limited activity against fungi(Guthrie, 1999).Its activity is reduced somewhat by 10% serum and to a greater extent by sulphydryl compounds, but is unaffected by 1% polysorbate or 0.1% lecithin. It has a half-life of about 96 daysat pH 8 and 25oC (Toler, 1985). Bronopol is most stable under acid conditons;the initial decomposition appears to involve the liberation of formaldehyde and the formulation of bromonitroethanol. A secondorder reaction involving bronopol and formaldehyde occurs simultaneously to produce 2-hydro-xymethyl-2-nitro-1,3-propanediol, which itself decomposes with the loss of formaldehyde. Bronopol has been employed extensively as a preservative for pharmaceuticalandcosmetic products.However, its use to preserve products containing secondary amines should be avoided as the by-product of this reaction is nitrosoamine which is carcinogenic. Details of the microbiological activity,chemical stability,toxicology and uses of bronopol are documented by Bryce et al. (1978),Croshaw & Holland (1984),Toler (1985) and Rossmorc and Sondossi (1988). Dcnyer and Wallhausser (1990) have provided useful information about bronopol, the typical in-use concentration of which is 0.01-0.1% w/v. Sulphhydryl compounds act as appropriate neutralizers inpreservative efficacy tests. |
|
General Description |
White crystals. Ignite easily and burn readily. May detonate under strong shock. Decomposes when heated, evolving toxic gases. Toxic by skin absorption, inhalation or ingestion. |
|
Air & Water Reactions |
Highly flammable. Water soluble. |
|
Reactivity Profile |
Incompatible with strong oxidizing agents, strong bases, strong reducing agents, acid chlorides and acid anhydrides. 2-Bromo-2-nitro-1,3-propanediol is also incompatible with sulfhydryl compounds or with aluminum or iron containers (it is stable in contact with tin or stainless steel). |
|
Hazard |
Toxic by all routes of exposure; skin irritant. |
|
Health Hazard |
Fire may produce irritating and/or toxic gases. Contact may cause burns to skin and eyes. Contact with molten substance may cause severe burns to skin and eyes. Runoff from fire control may cause pollution. |
|
Fire Hazard |
Flammable/combustible material. May be ignited by friction, heat, sparks or flames. Some may burn rapidly with flare burning effect. Powders, dusts, shavings, borings, turnings or cuttings may explode or burn with explosive violence. Substance may be transported in a molten form at a temperature that may be above its flash point. May re-ignite after fire is extinguished. |
|
Pharmaceutical Applications |
Bronopol 0.01–0.1% w/v is used as an antimicrobial preservative either alone or in combination with other preservatives in topical pharmaceutical formulations, cosmetics, and toiletries; the usual concentration is 0.02% w/v. |
|
Contact allergens |
Bronopol is a preservative sometimes considered as a formaldehyde releaser. It was reported to be an allergen in cosmetics, cleaning agents, dairy workers, and a lubricant jelly used for ultrasound examination.https://www.smartpractice.com https://www.contactdermatitisinstitute.com |
|
Safety Profile |
Poison by ingestion, subcutaneous, intravenous, and intraperitoneal routes. Moderately toxic by skin contact. An eye and human skin irritant. An antiseptic. When heated to decomposition it emits very toxic fumes of NOx, and Br-. |
|
Safety |
Bronopol is used widely in topical pharmaceutical formulations and cosmetics as an antimicrobial preservative. Although bronopol has been reported to cause both irritant and hypersensitivity adverse reactions following topical use, it is generally regarded as a nonirritant and nonsensitizing material at concentrations up to 0.1% w/v. At a concentration of 0.02% w/v, bronopol is frequently used as a preservative in ‘hypoallergenic’ formulations. Animal toxicity studies have shown no evidence of phototoxicity or tumor occurrence when bronopol is applied to rodents topically or administered orally; and there is no in vitro or in vivo evidence of mutagenicity; this is despite the demonstrated potential of bronopol to liberate nitrite on decomposition, which in the presence of certain amines may generate nitrosamines. Formation of nitrosamines in formulations containing amines may be reduced by limiting the concentration of bronopol to 0.01% w/v and including an antioxidant such as 0.2% w/v alpha tocopherol or 0.05% w/v butylated hydroxytoluene;(14) other inhibitor systems may also be appropriate. LD50 (dog, oral): 250 mg/kg LD50 (mouse, IP): 15.5 mg/kg LD50 (mouse, IV): 48 mg/kg LD50 (mouse, oral): 270 mg/kg LD50 (mouse, SC): 116 mg/kg LD50 (mouse, skin): 4.75 g/kg LD50 (rat, IP): 26 mg/kg LD50 (rat, IV): 37.4 mg/kg LD50 (rat, oral): 180 mg/kg LD50 (rat, SC): 170 mg/kg LD50 (rat, skin): 1.6 g/kg |
|
storage |
Bronopol is stable and its antimicrobial activity is practically unaffected when stored as a solid at room temperature and ambient relative humidity for up to 2 years. The pH of a 1.0% w/v aqueous solution is 5.0–6.0 and falls slowly during storage; solutions are more stable in acid conditions. Microbiological assay results indicate longer half-lives than those obtained by HPLC and thus suggest that degradation products may contribute to antimicrobial activity. Formaldehyde and nitrites are among the decomposition products, but formaldehyde arises in such low concentrations that its antimicrobial effect is not likely to be significant. On exposure to light, especially under alkaline conditions, solutions become yellow or brown-colored but the degree of discoloration does not directly correlate with loss of antimicrobial activity. The bulk material should be stored in a well-closed, nonaluminum container protected from light, in a cool, dry place. |
|
Incompatibilities |
Sulfhydryl compounds cause significant reductions in the activity of bronopol, and cysteine hydrochloride may be used as the deactivating agent in preservative efficacy tests; lecithin/polysorbate combinations are unsuitable for this purpose. Bronopol is incompatible with sodium thiosulfate, with sodium metabisulfite, and with amine oxide or protein hydrolysate surfactants. Owing to an incompatibility with aluminum, the use of aluminum in the packaging of products that contain bronopol should be avoided. |
|
Regulatory Status |
Included in topical pharmaceutical formulations licensed in Europe. Included in the Canadian List of Acceptable Non-medicinal Ingredients. |
InChI:InChI=1/C3H6BrNO4/c4-3(1-6,2-7)5(8)9/h6-7H,1-2H2
52-51-7 Relevant articles
Conformational transformations and autooxidation of 5-bromo-2-(2-methylpropyl)-5-nitro-1,3,2-dioxaborinane
Valiakhmetova, O. Yu.,Tyumkina,Meshcheryakova,Khalilov,Kuznetsov
, p. 926 - 931 (2017)
Conformational study of 5-bromo-2-(2-met...
New reaction of the sodium salt of 2-nitroethanol. X-ray analysis of the sodium salt of 2-oxo-3-hydroxypropionic acid oxime, 2-bromo-2-nitropropane-1,3-diol, and the model 2,2-dinitropropane-1,3-diol
Fedorov,Golovina,Arakcheeva,Trofimova,Atovmyan
, p. 1157 - 1161 (1996)
A novel reaction of the sodium salt of 2...
Preparation method of bronopol
-
Paragraph 0044; 0047-0048, (2021/04/21)
The invention discloses a preparation me...
A PROCESS FOR THE PREPARATION OF BRONOPOL
-
Page/Page column 22-24, (2009/10/21)
The invention provides a process for pre...
Method and composition for preserving antigens and process for utilizing cytological material produced by same
-
, (2008/06/13)
A method and composition for fixing and ...
52-51-7 Process route

-
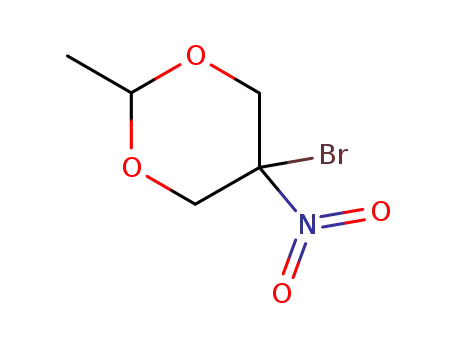
- 53983-00-9
5-bromo-2-methyl-5-nitro-1,3-dioxane

-
- 52-51-7
2-bromo-2-nitro-1,3-propanediol
| Conditions | Yield |
|---|---|
|
|
83.1% |
-
-
C3H5Cl2NO2

-

- 52-51-7
2-bromo-2-nitro-1,3-propanediol
| Conditions | Yield |
|---|---|
|
With water; phosphorus pentabromide; In ethanol; at 60 ℃; for 48h; under 0 Torr;
|
85% |
52-51-7 Upstream products
-
50-00-0

formaldehyd
-
563-70-2
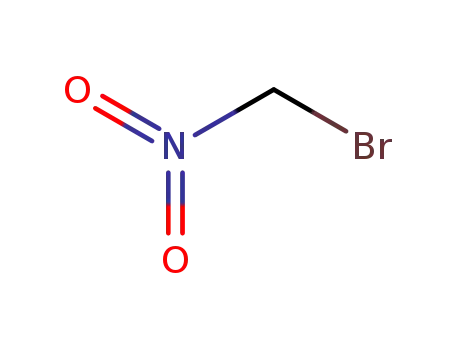
bromonitromethane
-
126-11-4
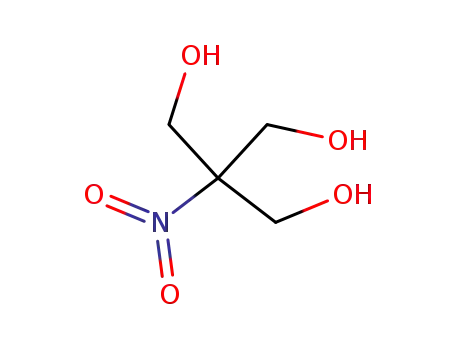
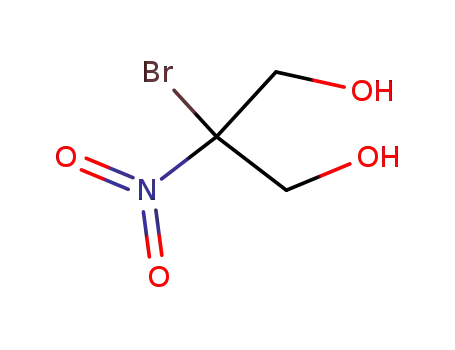
2-hydroxymethyl-2-nitro-propane-1,3-diol
-
75-52-5
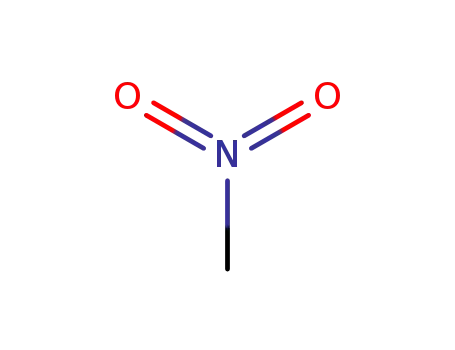
nitromethane
52-51-7 Downstream products
-
103281-09-0
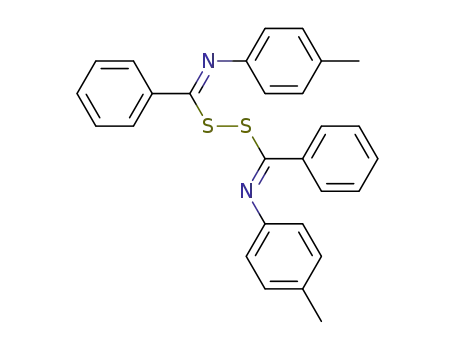
bis-(N-p-tolyl-benzimidoyl)-disulfane
-
103282-28-6
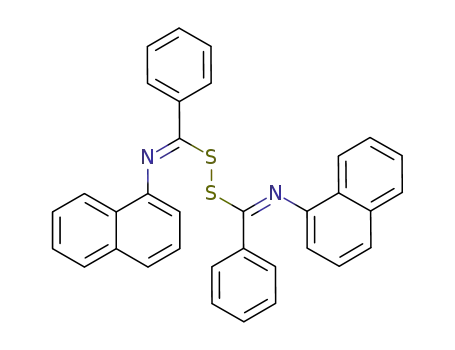
bis-(N-[1]naphthyl-benzimidoyl)-disulfane
-
5437-60-5
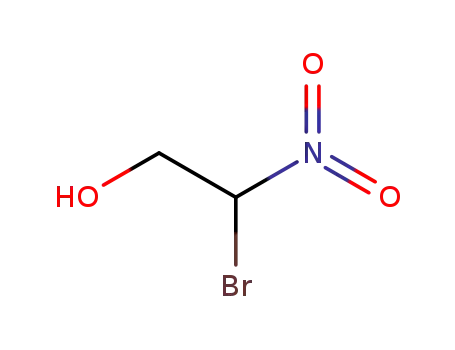
2-bromo-2-nitro-1-ethanol
-
464-10-8
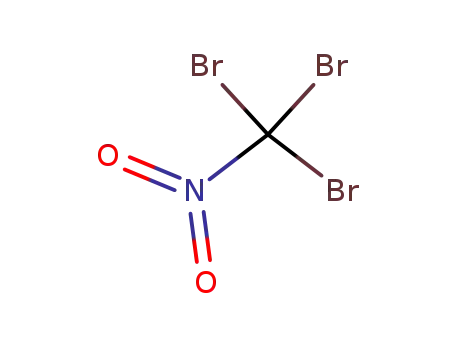
bromopicrin
Relevant Products
-
5-Bromo-5-Nitro-1,3-Dioxane
CAS:30007-47-7
-
5-(2′-Hydroxy-3′-Naphthamide)-2-Benzimidazolone
CAS:26848-40-8
-
Cetearyl Alcohol
CAS:67762-27-0