7647-15-6
- Product Name:Sodium Bromide
- Molecular Formula:NaBr
- Purity:99%
- Molecular Weight:102.894
Product Details;
CasNo: 7647-15-6
Molecular Formula: NaBr
Appearance: white powder
Factory supply good quality Sodium Bromide 7647-15-6 with stock
- Molecular Formula:NaBr
- Molecular Weight:102.894
- Appearance/Colour:white powder
- Melting Point:755 °C(lit.)
- Refractive Index:1.6412
- Boiling Point:1390 °C
- Flash Point:1390°C
- PSA:0.00000
- Density:3.203 g/cm3
- LogP:-2.99600
Sodium bromide(Cas 7647-15-6) Usage
|
Chemical Description |
Sodium bromide is a salt that is commonly used as a source of bromide ions in chemical reactions. |
|
Use Description |
Sodium bromide, a chemical compound, finds applications in various fields. In the medical industry, it has historically been used as a sedative and anticonvulsant, although its medical use has declined in favor of safer alternatives. In the oil and gas industry, sodium bromide is utilized as a dense brine fluid in drilling fluids, aiding in wellbore stability and preventing blowouts. In the photography sector, it plays a crucial role as a component in silver bromide emulsions used in traditional black-and-white photography. Additionally, sodium bromide is used in some chemical synthesis processes and as a disinfectant in water treatment, particularly in hot tubs and swimming pools. Its ability to control microbial growth in water makes it valuable for maintaining water quality. While its applications have evolved over time, sodium bromide continues to be relevant in these various fields for its unique properties and functions. |
|
Chemical Reactions |
Utilized in the electrochemical bromination of enamide derivatives, facilitating the formation of C(sp2)鈭払r bonds. |
|
Synthesis Reaction |
Synthesized by combining sodium (Na) and bromine (Br2), forming sodium bromide (NaBr) through a synthesis reaction. |
|
Surface Activity and Ionic Liquids |
Dodecylbenezenesulfonate-based anionic surface active ionic liquids (DBS-ILs) paired with onium cations have been synthesized. DBS-ILs exhibit high surface activity and can form micelles, which can be transformed into vesicles with the addition of sodium bromide (NaBr). |
InChI:InChI=1/BrH.Na/h1H;/q;+1/p-1
7647-15-6 Relevant articles
Gel Systems for the Belousov-Zhabotinskii Reaction
Yamaguchi, T.,Kuhnert, L.,Nagy-Ungvarai, Zs.,Mueller, S. C.,Hess, B.
, p. 5831 - 5837 (1991)
Gel systems and their advantages over an...
Evaluation of kinetic parameters for the thermal decomposition of γ-irradiated sodium bromate by dynamic thermogravimetry
Nair,Malayil, Koshy Kunju,Jacob, P.Daisamma
, p. 61 - 68 (1989)
The thermal decomposition of γ-irradiate...
Biochar and kinetics studies on the reduction of sodium bromate by a cobaloxime in an aqueous media: How we can remove a toxic substance from our environment
Celestine, Michael J.,Holder, Alvin A.,Kumar, Sandeep,Nunez, Brianne S.,Tano, Criszcele M.,Tonsel-White, Elizabeth A.
, (2020)
The reduction of sodium bromate (NaBrO3)...
Nitrogen-rich carbon nitride materials shock-synthesized from carbon tetrahalide and sodium dicyanoamide
Shibata, Kazusato,Sekine, Toshimori
, p. 501 - 505 (2006)
Shock reactions between CX4 (X=Br or I) ...
Ion Exchange of Layered Alkali Titanates (Na2Ti3O7, K2Ti4O9, and Cs2Ti5O11) with Alkali Halides by the Solid-State Reactions at Room Temperature
Ogawa, Makoto,Saothayanun, Taya Ko,Sirinakorn, Thipwipa Tip
, p. 4024 - 4029 (2020/04/08)
Ion exchange of layered alkali titanates...
Nitryl cyanide, NCNO2
Rahm, Martin,Belanger-Chabot, Guillaume,Haiges, Ralf,Christe, Karl O.
supporting information, p. 6893 - 6897 (2014/07/08)
The elusive nitryl cyanide, NCNO2, has b...
Synthesis, characterization, and catalytic behavior of methoxy- and dimethoxy-substituted pyridinium-type ionic liquids
Manikandan, Chitrarasu,Ganesan, Kilivelu
, p. 3362 - 3367 (2014/12/11)
Synthesis of methoxy-substituted pyridin...
7647-15-6 Process route
-
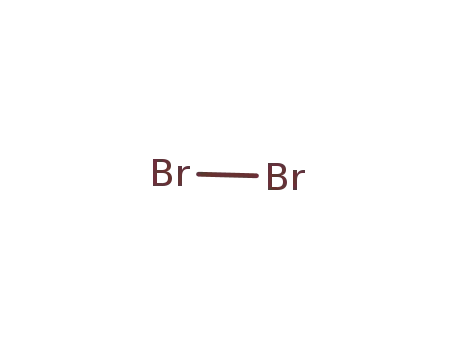
- 7726-95-6
bromine

-
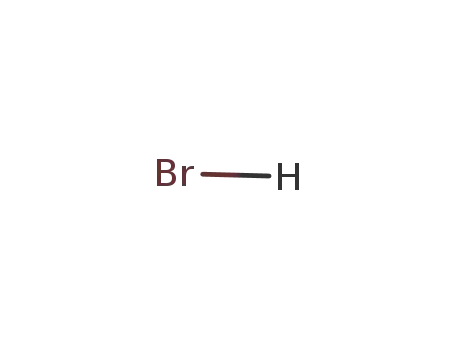
- 10035-10-6,12258-64-9
hydrogen bromide

-
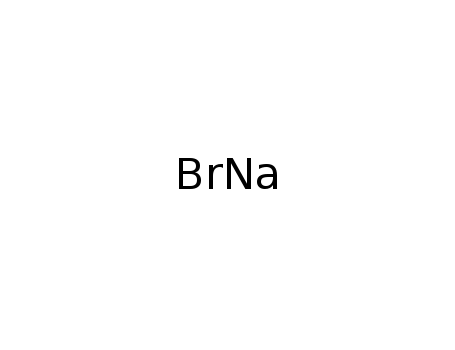
- 7647-15-6
sodium bromide
| Conditions | Yield |
|---|---|
|
With sodium azide; water; In water; byproducts: H2O, N2;
|
|
|
With NaN3; H2O; In water; byproducts: H2O, N2;
|
-
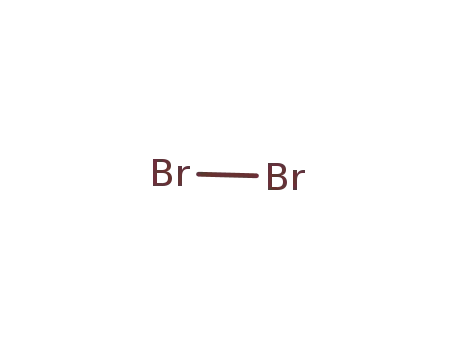
- 7726-95-6
bromine

-
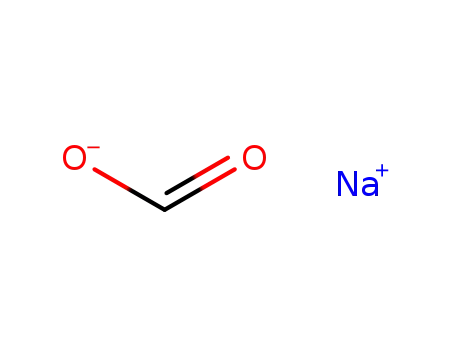
- 141-53-7
sodium formate

-
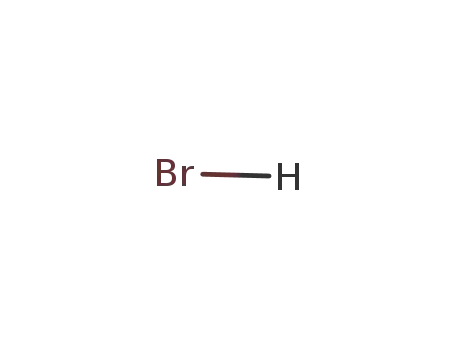
- 10035-10-6,12258-64-9
hydrogen bromide

-

- 7647-15-6
sodium bromide
| Conditions | Yield |
|---|---|
|
byproducts: CO2; fast reaction in the presence of ZnO;
|
7647-15-6 Upstream products
-
134509-56-1
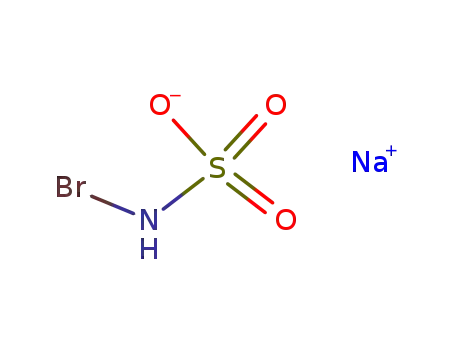
N-bromosulfamic acid sodium salt
-
10035-10-6
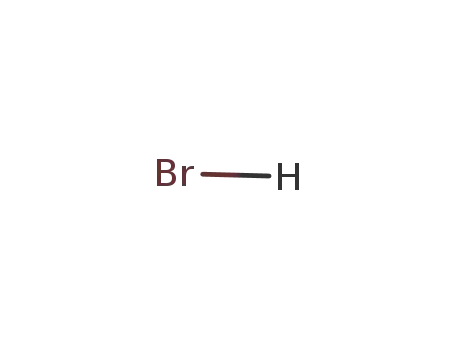
hydrogen bromide
-
773837-37-9
sodium cyanide
-
7726-95-6
bromine
7647-15-6 Downstream products
-
7787-70-4

copper(I) bromide
-
7726-95-6
bromine
-
7440-23-5

sodium
-
7647-14-5

sodium chloride
Relevant Products
-
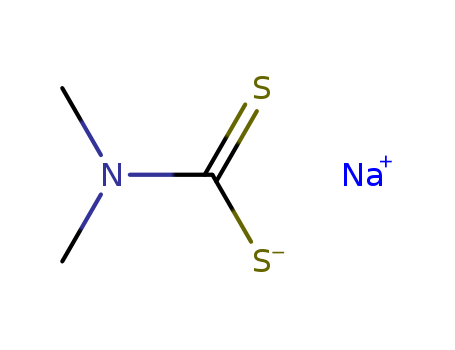
Sodium dimethyldithiocarbamate
CAS:128-04-1
-
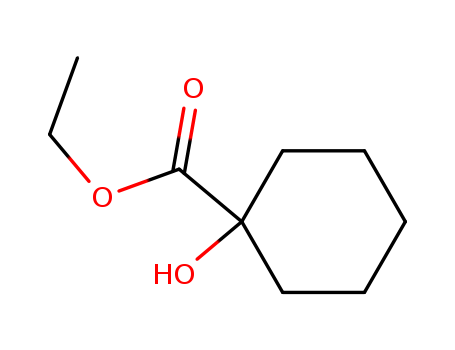
1-Hydroxy-1-Cyclohexylmethanecarboxylate
CAS:1127-01-1
-
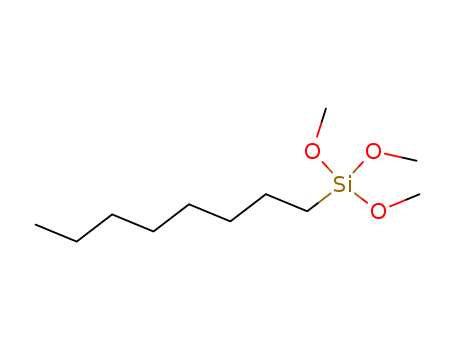
Mercaptopropyl Trimethoxysilane
CAS:3069-40-7