6294-17-3
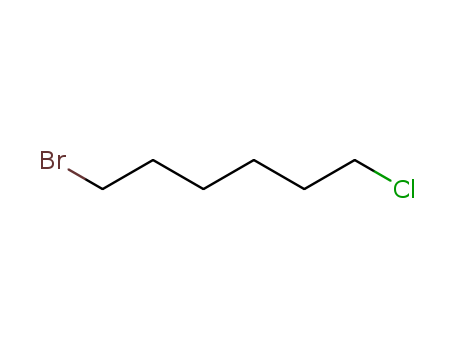
- Product Name:1-Bromo-6-Chlorohexane
- Molecular Formula:C6H12BrCl
- Purity:99%
- Molecular Weight:199.518
Product Details;
CasNo: 6294-17-3
Molecular Formula: C6H12BrCl
Manufacturer supply 1-Bromo-6-Chlorohexane 6294-17-3 with sufficient stock and high standard
- Molecular Formula:C6H12BrCl
- Molecular Weight:199.518
- Vapor Pressure:0.196mmHg at 25°C
- Refractive Index:n20/D 1.481(lit.)
- Boiling Point:217.4 °C at 760 mmHg
- Flash Point:101.1 °C
- PSA:0.00000
- Density:1.311 g/cm3
- LogP:3.18050
1-Bromo-6-chlorohexane(Cas 6294-17-3) Usage
InChI:InChI=1/C6H12BrCl/c7-5-3-1-2-4-6-8/h1-6H2
6294-17-3 Relevant articles
A novel stereoselective one-pot conversion of alcohols into alkyl halides mediated by N,N′-diisopropylcarbodiimide
Crosignani, Stefano,Nadal, Brice,Li, Zhengning,Linclau, Bruno
, p. 260 - 261 (2007/10/03)
Alcohols can be converted in high yields...
Process for the preparation of α-bromo, ω-chloroalkanes
-
, (2008/06/13)
Described is a process for easily prepar...
Imine-Directed Metalation of o-Tolualdehyde: The Use of Catalytic Amine Base. A Route to 2-(8-Phenyloctyl)benzaldehyde
Forth, Michael A.,Mitchell, Michael B.,Smith, Stephen A. C.,Gombatz, Kerry,Snyder, Lawrence
, p. 2616 - 2619 (2007/10/02)
-
A New and Efficient One-Pot Preparation of Alkyl Halides From Alcohols
Camps, Francisco,Gasol, Vicens,Guerrero, Angel
, p. 511 - 512 (2007/10/02)
Primary alkanols and 2-alkenols are conv...
6294-17-3 Process route
-
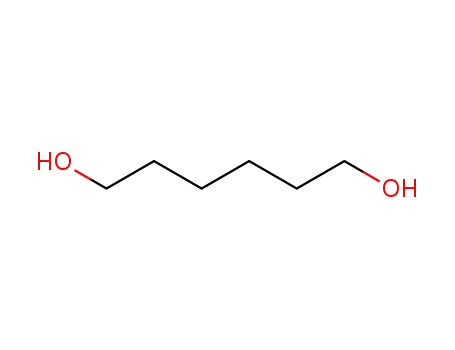
- 629-11-8
1,6-hexanediol

-
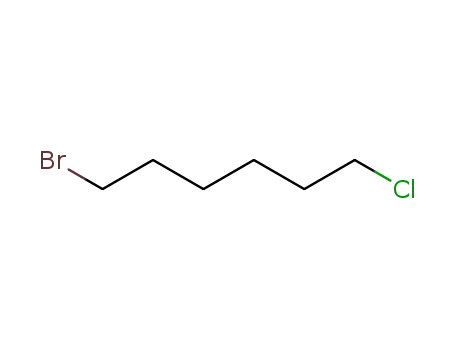
- 6294-17-3
1-bromo-6-chlorohexane

-
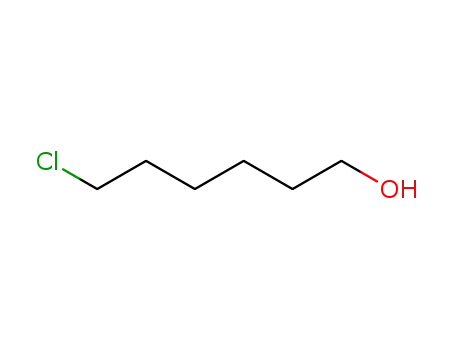
- 2009-83-8
6-chloro-1-hexanol
Conditions
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; sodium hydroxide; zinc(II) chloride;
|
34% |
-

- 2009-83-8
6-chloro-1-hexanol

-

- 6294-17-3
1-bromo-6-chlorohexane
Conditions
| Conditions | Yield |
|---|---|
|
6-chloro-1-hexanol; With diisopropyl-carbodiimide; copper(II) bis(trifluoromethanesulfonate); In tetrahydrofuran; at 100 ℃; for 0.0833333h; microwave irradiation;
With Acetyl bromide; In tetrahydrofuran; at 150 ℃; for 0.0833333h; Further stages.; microwave irradiation;
|
96% |
|
With phosphorus tribromide;
|
|
|
|
|
|
Multi-step reaction with 2 steps
1: tetrahydrofuran / 0.25 h / Ambient temperature
2: LiBr / tetrahydrofuran; hexamethylphosphoric acid triamide / 3 h / Heating
With lithium bromide; In tetrahydrofuran; N,N,N,N,N,N-hexamethylphosphoric triamide;
|
|
|
|
6294-17-3 Upstream products
-
2009-83-8

6-chloro-1-hexanol
-
544-10-5
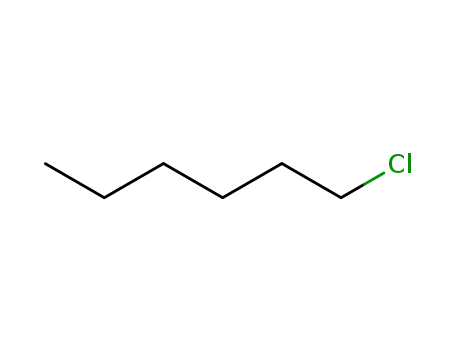
1-Chlorohexane
-
4286-55-9
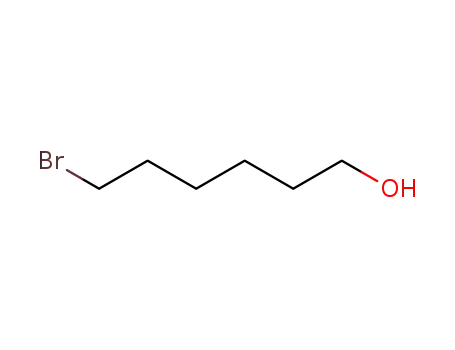
1-bromo-6-hexanol
-
629-11-8

1,6-hexanediol
6294-17-3 Downstream products
-
928-89-2
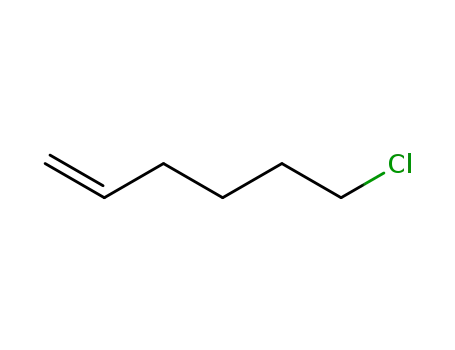
6-chlorohexene
-
24088-97-9
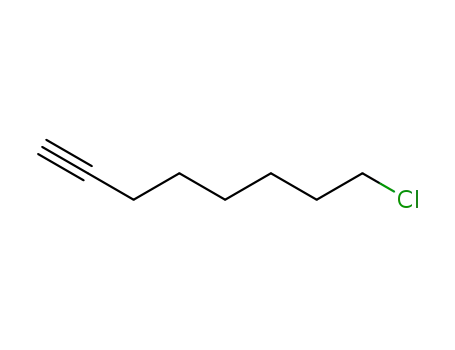
8-chloro-oct-1-yne
-
110718-81-5
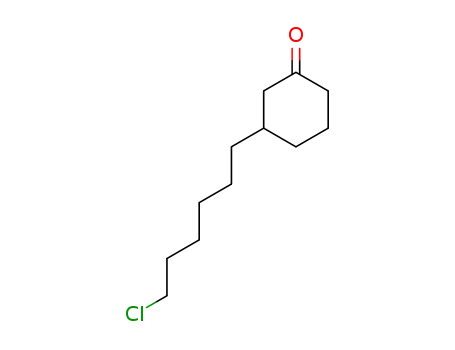
3-(6-chlorohexyl)cyclohexanone
-
139653-26-2
6-<(6-chlorohexyl)oxy>flavone
Relevant Products
-
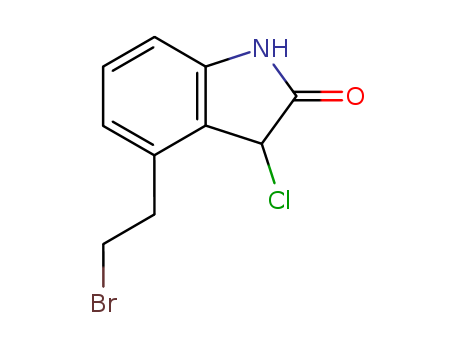
4-(2'-Bromoethyl)-3-Chloro-1,3-Dihydro-2-Indolinone
CAS:120427-95-4
-
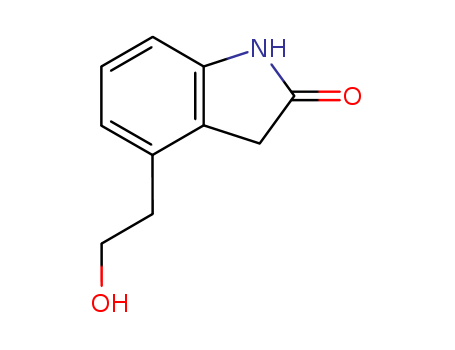
4-(2'-Hydroxyethyl)-2-Indolinone
CAS:139122-19-3
-
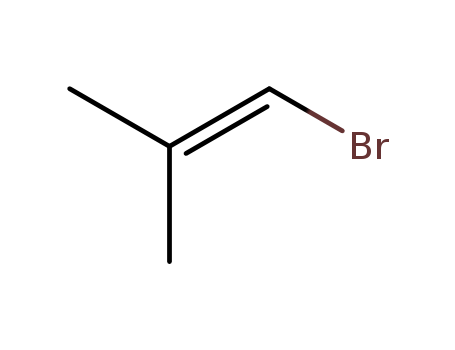
1-Bromo-2-Methylpropene
CAS:3017-69-4