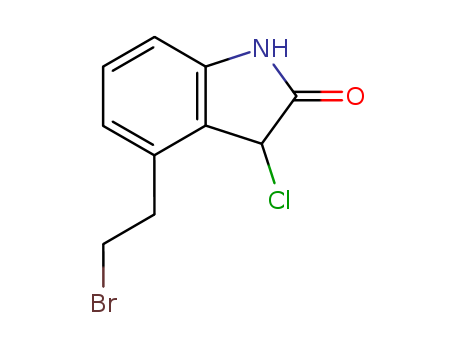
120427-95-4
- Product Name:4-(2'-Bromoethyl)-3-Chloro-1,3-Dihydro-2-Indolinone
- Molecular Formula:C10H9BrClNO
- Purity:99%
- Molecular Weight:274.545
Product Details;
CasNo: 120427-95-4
Molecular Formula: C10H9BrClNO
Factory Export Top Purity 4-(2'-Bromoethyl)-3-Chloro-1,3-Dihydro-2-Indolinone 120427-95-4 In Stock
- Molecular Formula:C10H9BrClNO
- Molecular Weight:274.545
- Vapor Pressure:0mmHg at 25°C
- Refractive Index:1.633
- Boiling Point:374.736 °C at 760 mmHg
- PKA:12.08±0.40(Predicted)
- Flash Point:180.434 °C
- PSA:29.10000
- Density:1.647 g/cm3
- LogP:2.99400
4-(2'-BROMOETHYL)-3-CHLORO-1,3-DIHYDRO-2H-INDOLE-2-ONE(Cas 120427-95-4) Usage
InChI:InChI=1/C10H9BrClNO/c11-5-4-6-2-1-3-7-8(6)9(12)10(14)13-7/h1-3,9H,4-5H2,(H,13,14)
120427-95-4 Relevant articles
Preparation method of 4-(2-bromoethyl)-1, 3-dihydro-2H-indole-2-ketone
-
Paragraph 0032; 0035; 0036; 0040; 0043-0044; 0048; 0051-0052, (2021/05/05)
The invention provides a preparation met...
PROCESS FOR PURIFICATION OF ROPINIROLE
-
Page/Page column 21-22, (2010/02/13)
The invention discloses an improved proc...
Development of large-scale syntheses of ropinirole in the pursuit of a manufacturing process
Hayler, John D.,Howie, Simon L. B.,Giles, Robert G.,Negus, Alan,Oxley, Paul W.,Walsgrove, Timothy C.,Whiter
, p. 3 - 9 (2013/09/08)
Two plant syntheses of ropinirole {4-[2-...
Some synthetic approaches to ropinirole (SK and F 101468-A): A potent dopamine receptor agonist
Hayler,Howie,Giles,Negus,Oxley,Walsgrove,Walsh,Dagger,Fortunak,Mastrocola
, p. 875 - 882 (2007/10/02)
Three new routes to ropinirole (SK and F...
120427-95-4 Process route
-
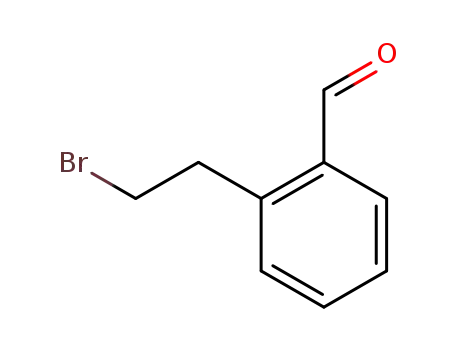
- 22901-09-3
2-(2-bromoethyl)benzaldehyde

-
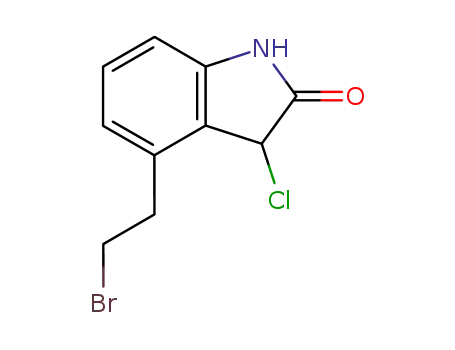
- 120427-95-4
4-(2-bromoethyl)-3-chloro-1,3-dihydro-2H-indolin-2-one
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: 80 percent / MeONa / methanol / 1 h / 0 °C
2: 53 percent / AcCl, FeCl3 / CH2Cl2 / 3 h / 0 - 5 °C
With sodium methylate; iron(III) chloride; acetyl chloride; In methanol; dichloromethane;
|
-
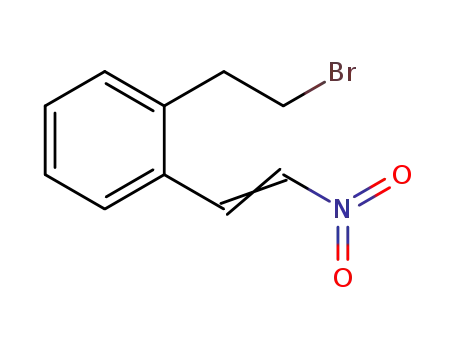
- 120427-94-3
2-(2-bromoethyl)-β-nitrostyrene

-

- 120427-95-4
4-(2-bromoethyl)-3-chloro-1,3-dihydro-2H-indolin-2-one
| Conditions | Yield |
|---|---|
|
With iron(III) chloride; acetyl chloride; In dichloromethane; at 0 - 5 ℃; for 3h;
|
83.9% |
120427-95-4 Upstream products
-
120427-94-3
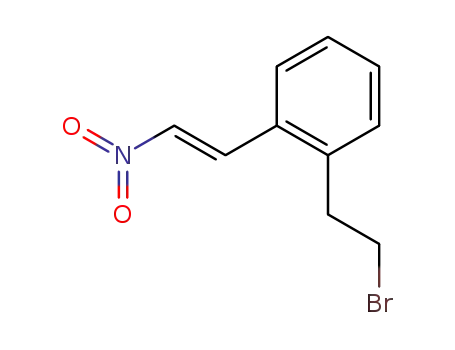
2-(2-bromoethyl)-β-nitrostyrene
-
22901-09-3

2-(2-bromoethyl)benzaldehyde
-
75-36-5
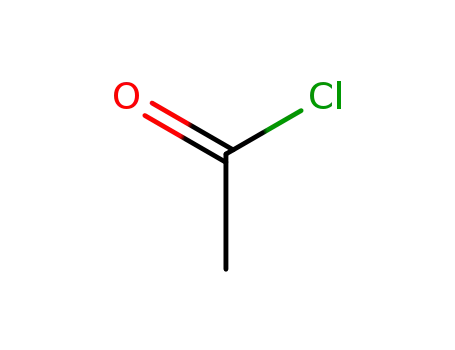
acetyl chloride
-
493-05-0
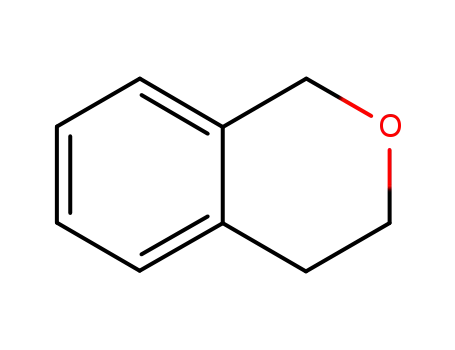
isochromane
120427-95-4 Downstream products
-
120427-96-5
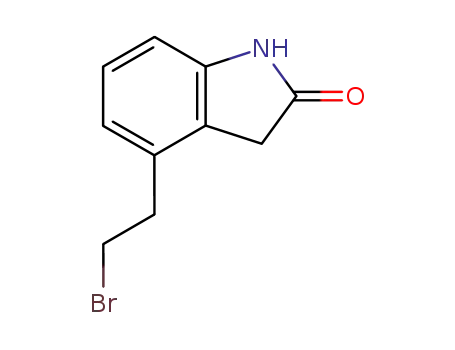
4-(2'-bromoethyl)-1,3-dihydro-2H-indol-2-one
-
91374-21-9
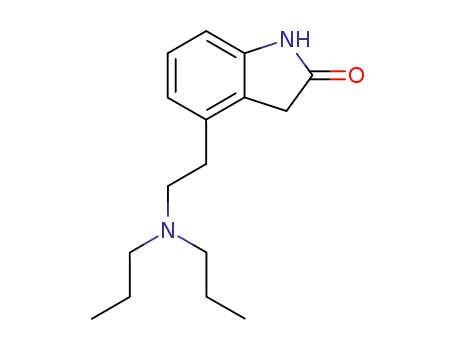
Ropinirole
-
120427-93-2
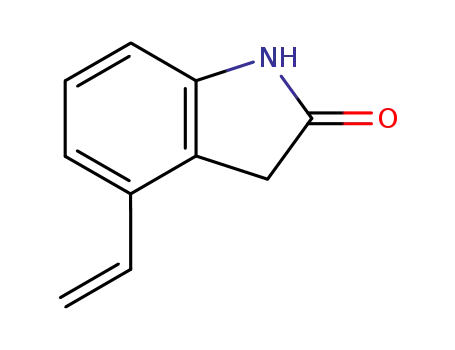
4-ethylenyl-1,3-dihydro-2H-indol-2-one
-
139122-19-3
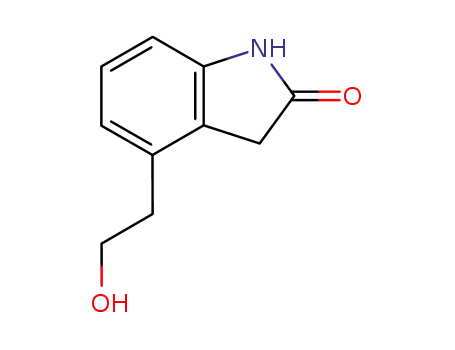
4-(2'-hydroxyethyl)-1,3-dihydro-2H-indol-2-one
Relevant Products
-
1-Bromo-6-Chlorohexane
CAS:6294-17-3
-
4-(2'-Hydroxyethyl)-2-Indolinone
CAS:139122-19-3
-
1-Bromo-2-Methylpropene
CAS:3017-69-4