60421-23-0
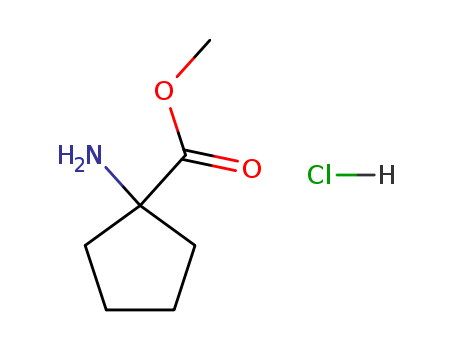
- Product Name:1-Amino-1-Cyclopentylmethanecarboxylate Hydrochloride
- Molecular Formula:C7H13 N O2 . Cl H
- Purity:99%
- Molecular Weight:179.647
Product Details;
CasNo: 60421-23-0
Molecular Formula: C7H13 N O2 . Cl H
Factory Sells Best Quality 1-Amino-1-Cyclopentylmethanecarboxylate Hydrochloride 60421-23-0 with stock
- Molecular Formula:C7H13 N O2 . Cl H
- Molecular Weight:179.647
- Vapor Pressure:0.653mmHg at 25°C
- Melting Point:207-208 °C (decomp)
- Boiling Point:186.7 °C at 760 mmHg
- Flash Point:58.7 °C
- PSA:52.32000
- Density:1.081g/cm3
- LogP:1.93320
Methyl 1-amino-1-cyclopentanecarboxylate hydrochloride(Cas 60421-23-0) Usage
|
General Description |
Methyl 1-amino-1-cyclopentanecarboxylate hydrochloride is a chemical compound with the molecular formula C7H12ClNO2. It is a salt form of the organic compound methyl 1-amino-1-cyclopentanecarboxylate, which is commonly used in the synthesis of various pharmaceuticals and agrochemicals. The hydrochloride salt form increases the solubility and stability of the compound, making it more suitable for use in pharmaceutical formulations. Methyl 1-amino-1-cyclopentanecarboxylate hydrochloride is a versatile building block in organic synthesis and is commonly used in the production of active pharmaceutical ingredients. It is also used as a chiral auxiliary in asymmetric synthesis to control the stereochemistry of reactions. |
InChI:InChI=1/C7H13NO2/c1-10-6(9)7(8)4-2-3-5-7/h2-5,8H2,1H3
60421-23-0 Relevant articles
Hydrogen-Borrowing Alkylation of 1,2-Amino Alcohols in the Synthesis of Enantioenriched γ-Aminobutyric Acids
Hall, Christopher J. J.,Goundry, William R. F.,Donohoe, Timothy J.
supporting information, p. 6981 - 6985 (2021/03/01)
For the first time we have been able to ...
Distal Stereocontrol Using Guanidinylated Peptides as Multifunctional Ligands: Desymmetrization of Diarylmethanes via Ullman Cross-Coupling
Kim, Byoungmoo,Chinn, Alex J.,Fandrick, Daniel R.,Senanayake, Chris H.,Singer, Robert A.,Miller, Scott J.
supporting information, p. 7939 - 7945 (2016/07/07)
We report the development of a new class...
INHIBITORS OF HEPATITIS C VIRUS POLYMERASE
-
Paragraph 409; 410; 414, (2016/10/11)
The present invention provides, among ot...
A COMPOUND FOR INHIBITING 11B-HYDROXY STEROID DEHYDROGENASE 1, AND A PHARMACEUTICAL COMPOSITION COMPRISING THE SAME
-
Paragraph 0449-0451, (2014/08/06)
Disclosed are a novel compound or a phar...
60421-23-0 Process route
-
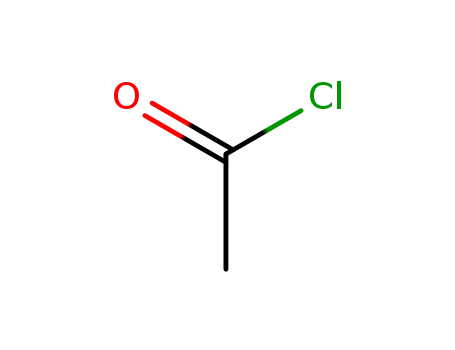
- 75-36-5
acetyl chloride

-
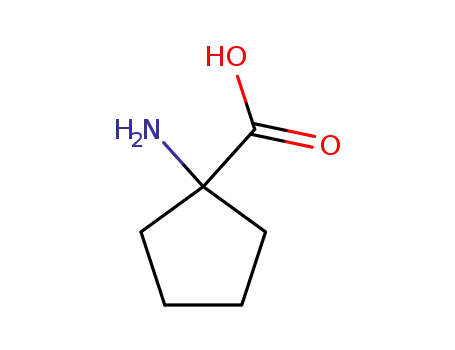
- 52-52-8
1-amino-1-cyclopentanecarboxylic acid

-
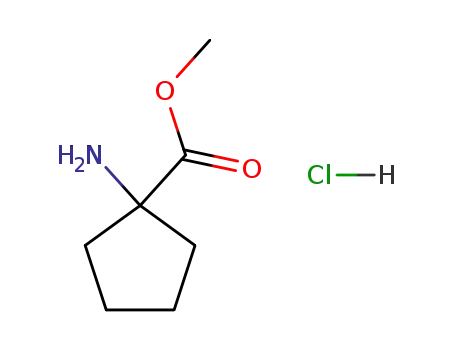
- 60421-23-0
cycloleucine methyl ester hydrochloride
| Conditions | Yield |
|---|---|
|
acetyl chloride; With methanol; at 20 ℃;
1-amino-1-cyclopentanecarboxylic acid; In methanol; at 20 ℃;
|
-

- 52-52-8
1-amino-1-cyclopentanecarboxylic acid

-

- 60421-23-0
cycloleucine methyl ester hydrochloride
| Conditions | Yield |
|---|---|
|
|
100% |
|
In methanol;
|
60421-23-0 Upstream products
-
67-56-1
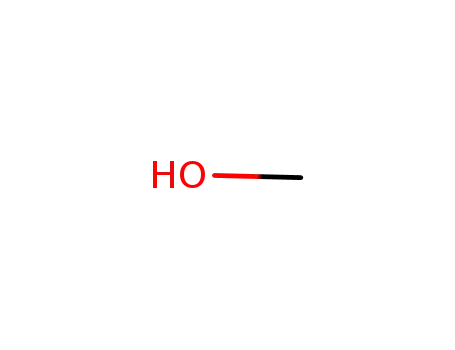
methanol
-
52-52-8

1-amino-1-cyclopentanecarboxylic acid
-
248262-96-6
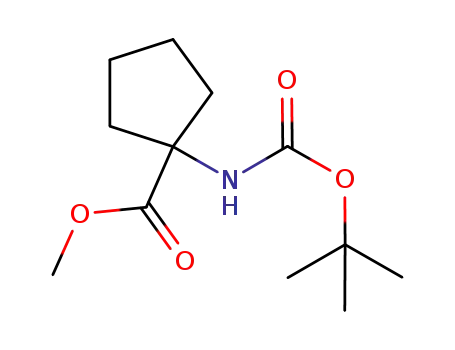
methyl 1-((tert-butoxycarbonyl)amino)cyclopentanecarboxylate
-
1001426-16-9
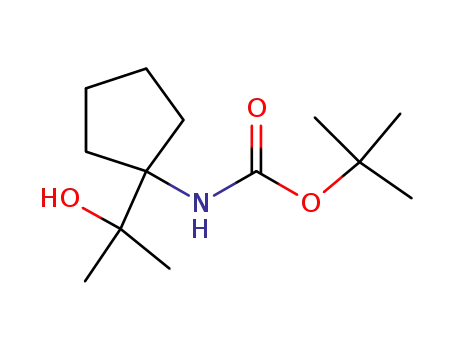
[1-(1-hydroxy-1-methylethyl)cyclopentyl]carbamic acid tert-butyl ester
60421-23-0 Downstream products
-
139456-08-9
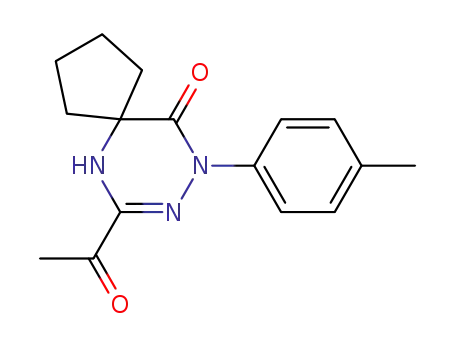
7-Acetyl-9-p-tolyl-6,8,9-triaza-spiro[4.5]dec-7-en-10-one
-
92398-62-4
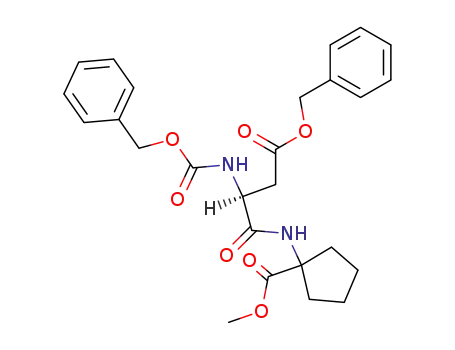
N-(benzyloxycarbonyl)-β-benzyl-L-aspartyl-α-aminocyclopentanecarboxylic acid methyl ester
-
139456-09-0
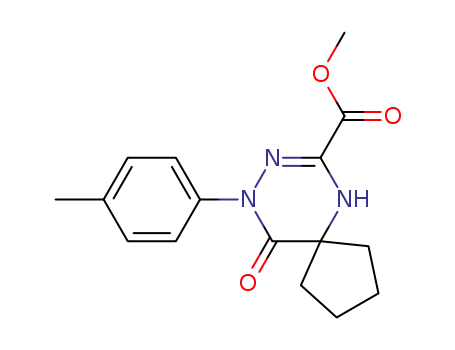
10-Oxo-9-p-tolyl-6,8,9-triaza-spiro[4.5]dec-7-ene-7-carboxylic acid methyl ester
-
139456-10-3
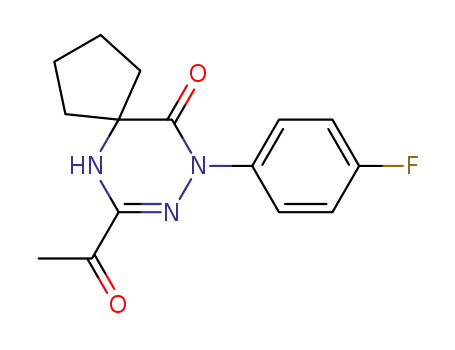
7-Acetyl-9-(4-fluoro-phenyl)-6,8,9-triaza-spiro[4.5]dec-7-en-10-one
Relevant Products
-
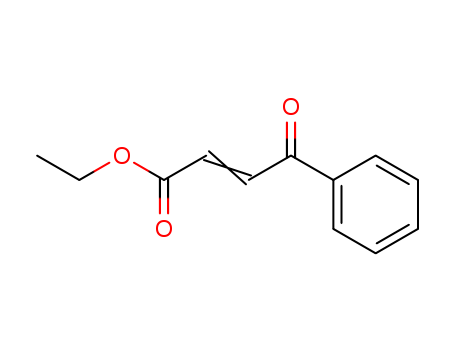
3-Benzoylpropyl Ethyl Ester
CAS:17450-56-5
-
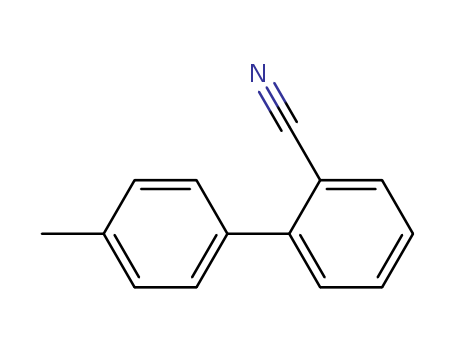
2-Methyl-2-Cyano Biphenyl
CAS:114772-53-1