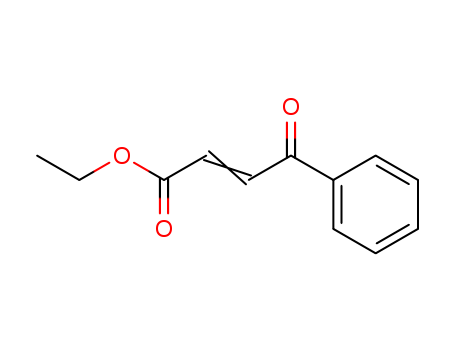
17450-56-5
- Product Name:3-Benzoylpropyl Ethyl Ester
- Molecular Formula:C12H12O3
- Purity:99%
- Molecular Weight:204.225
Product Details;
CasNo: 17450-56-5
Molecular Formula: C12H12O3
Appearance: clear yellow liquid
Factory supply good quality 3-Benzoylpropyl Ethyl Ester 17450-56-5 with stock
- Molecular Formula:C12H12O3
- Molecular Weight:204.225
- Appearance/Colour:clear yellow liquid
- Vapor Pressure:0.000839mmHg at 25°C
- Melting Point:32 °C
- Refractive Index:1.542-1.546
- Boiling Point:305.1 °C at 760 mmHg
- Flash Point:132.3 °C
- PSA:43.37000
- Density:1.109 g/cm3
- LogP:1.98860
Ethyl 3-benzoylacrylate(Cas 17450-56-5) Usage
|
Synthesis Reference(s) |
Tetrahedron Letters, 13, p. 407, 1972 DOI: 10.1016/S0040-4039(01)84337-9 |
|
General Description |
Ethyl 3-benzoylacrylate (ethyl trans-3-benzoylacrylate) undergoes enantioselective guanidine catalyst promoted Michael addition reaction with dithranol to yield Michael adduct. |
InChI:InChI=1/C12H12O3/c1-2-15-12(14)9-8-11(13)10-6-4-3-5-7-10/h3-9H,2H2,1H3
17450-56-5 Relevant articles
Catalytic Synthesis of 1 H-2-Benzoxocins: Cobalt(III)-Carbene Radical Approach to 8-Membered Heterocyclic Enol Ethers
De Bruin, Bas,De Zwart, Felix J.,Li, Zirui,Mathew, Simon,Wolzak, Lukas A.,Zhou, Minghui
supporting information, p. 20501 - 20512 (2021/12/03)
The metallo-radical activation of ortho-...
Chiral Hydroxytetraphenylene-Catalyzed Asymmetric Conjugate Addition of Boronic Acids to Enones
Chai, Guo-Li,Sun, A-Qiang,Zhai, Dong,Wang, Juan,Deng, Wei-Qiao,Wong, Henry N.C.,Chang, Junbiao
supporting information, p. 5040 - 5045 (2019/07/03)
(S)-2,15-Br2-DHTP-catalyzed asymmetric c...
A robust multifunctional ligand-controlled palladium-catalyzed carbonylation reaction in water
Gao, Pei-Sen,Zhang, Kan,Yang, Ming-Ming,Xu, Shan,Sun, Hua-Ming,Zhang, Jin-Lei,Gao, Zi-Wei,Zhang, Wei-Qiang,Xu, Li-Wen
supporting information, p. 5074 - 5077 (2018/05/26)
A novel, hydrophilic and recyclable meth...
Synthesis of 1,5-benzodiazepine derivatives using p-toluenesulfonic acid as catalyst
Wang, Shasha,Hu, Lijuan,Cheng, Suyan,Wang, Lanzhi
, p. 419 - 424 (2015/01/30)
A series of substituted ethyl 4-oxo-4-ph...
17450-56-5 Process route
-
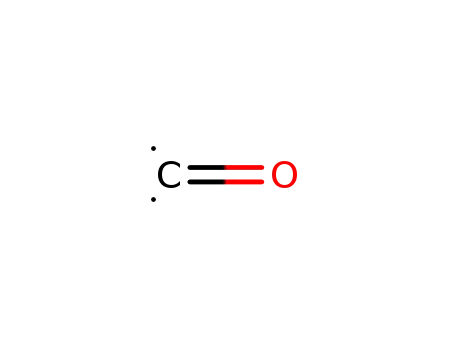
- 201230-82-2
carbon monoxide

-
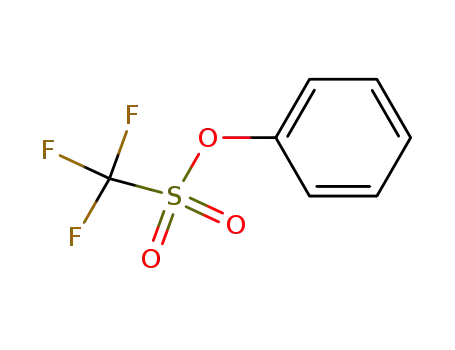
- 17763-67-6
Phenyl triflate

-
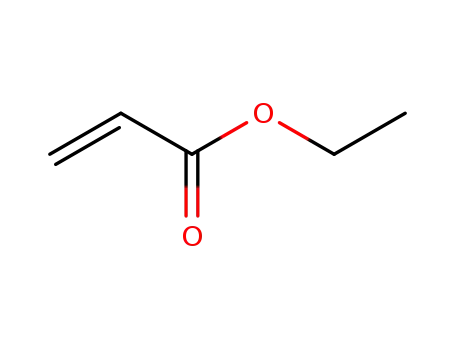
- 140-88-5,9003-32-1
ethyl acrylate

-
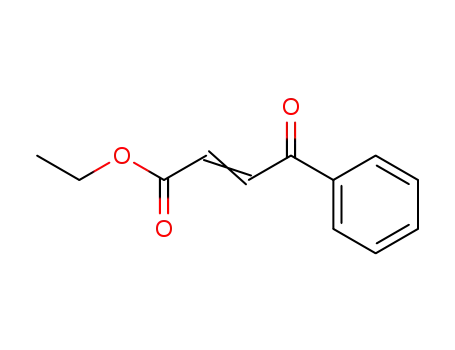
- 15121-89-8,20908-27-4,17450-56-5
ethyl 3-benzoyl acrylate
| Conditions | Yield |
|---|---|
|
With palladium diacetate; caesium carbonate; In water; at 35 - 95 ℃; under 3750.38 Torr; Schlenk technique; Inert atmosphere;
|
88% |
-
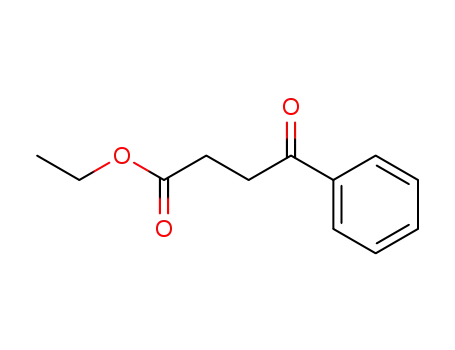
- 6270-17-3
ethyl 3-benzoylpropanoate

-

- 15121-89-8,20908-27-4,17450-56-5
ethyl 3-benzoyl acrylate
| Conditions | Yield |
|---|---|
|
With (Z/E)-2-bromo-2-[4-oxothiazolidin-2-ylidene]-N-phenylacetamide; dimethyl sulfoxide; for 1.5h; Reflux;
|
33% |
17450-56-5 Upstream products
-
64-17-5
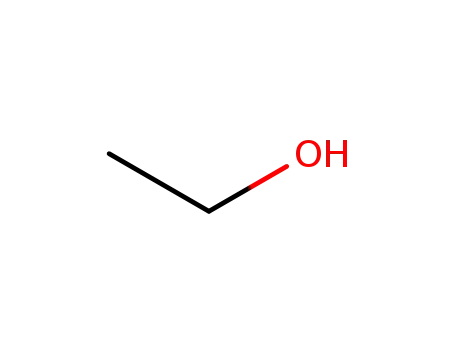
ethanol
-
583-06-2
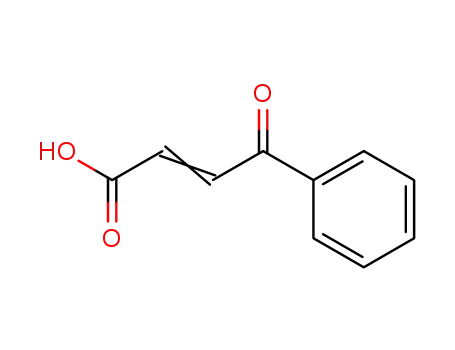
3-benzoylacrylic acid
-
33093-73-1
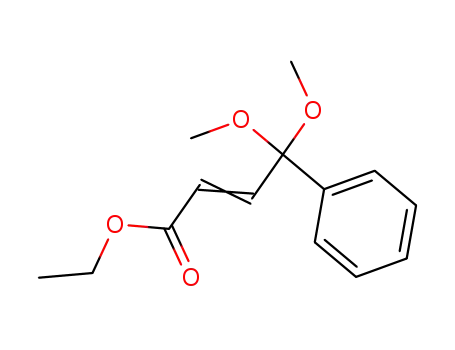
4,4-Dimethoxy-4-phenyl-crotonsaeure-aethylester
-
91497-49-3
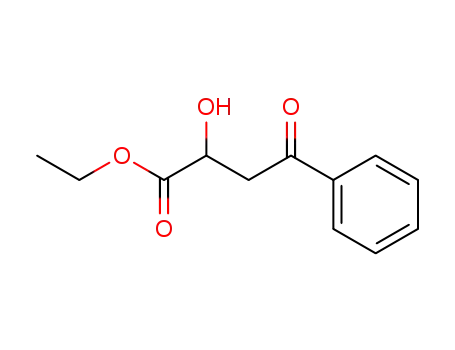
4-phenyl-2-hydroxy-4-oxobutyric acid ethyl ester
17450-56-5 Downstream products
-
95317-68-3
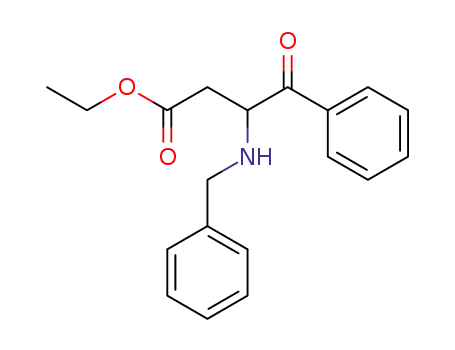
3-benzylamino-4-oxo-4-phenyl-butyric acid ethyl ester
-
3448-48-4
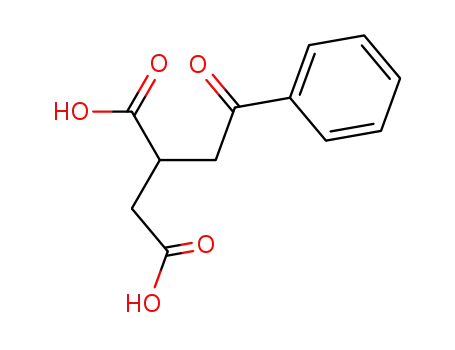
phenacyl-succinic acid
-
583-06-2

3-benzoylacrylic acid
-
80276-25-1
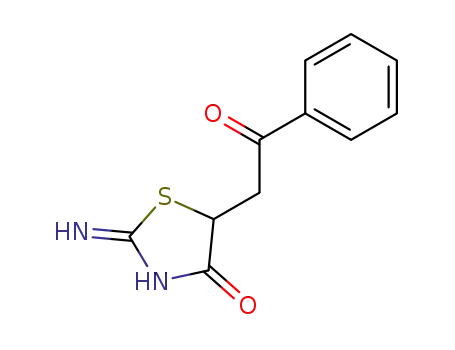
2-imino-5-(2-phenyl-2-oxoethyl)-4-oxo-1,3-thiazolidine
Relevant Products
-
5-(2′-Hydroxy-3′-Naphthamide)-2-Benzimidazolone
CAS:26848-40-8
-
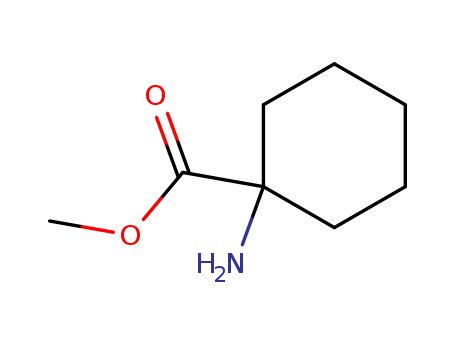
1-Amino-1-Cyclohexylmethanecarboxylate
CAS:4507-57-7
-
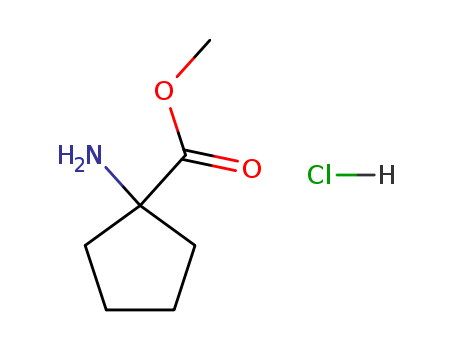
1-Amino-1-Cyclopentylmethanecarboxylate Hydrochloride
CAS:60421-23-0