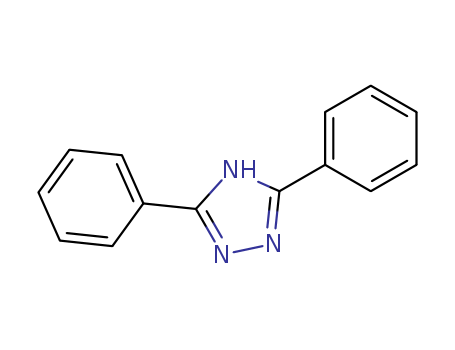
2039-06-7
- Product Name:3,5-Diphenyl-1-H-1,2,4-Triazole
- Molecular Formula:C14H11N3
- Purity:99%
- Molecular Weight:221.261
Product Details;
CasNo: 2039-06-7
Molecular Formula: C14H11N3
Manufacturer supply top purity 3,5-Diphenyl-1-H-1,2,4-Triazole 2039-06-7 with GMP standards
- Molecular Formula:C14H11N3
- Molecular Weight:221.261
- Vapor Pressure:6.1E-08mmHg at 25°C
- Melting Point:192°C
- Refractive Index:1.5770 (estimate)
- Boiling Point:440°Cat760mmHg
- PKA:9.46±0.40(Predicted)
- Flash Point:206.8°C
- PSA:41.57000
- Density:1.197g/cm3
- LogP:3.13870
3,5-Diphenyl-4H-1,2,4-triazole(Cas 2039-06-7) Usage
|
Synthesis Reference(s) |
Tetrahedron, 26, p. 2619, 1970 DOI: 10.1016/S0040-4020(01)92836-4 |
InChI:InChI=1/C14H11N3/c1-3-7-11(8-4-1)13-15-14(17-16-13)12-9-5-2-6-10-12/h1-10H,(H,15,16,17)
2039-06-7 Relevant articles
C-(β-d-Glucopyranosyl)formamidrazones, formic acid hydrazides and their transformations into 3-(β-d-glucopyranosyl)-5-substituted-1,2,4- triazoles: A synthetic and computational study
Bokor, éva,Fekete, Attila,Varga, Gergely,Szocs, Béla,Czifrák, Katalin,Komáromi, István,Somsák, László
, p. 10391 - 10404 (2013)
Synthesis of O-perbenzoylated 3-(β-d-glu...
PEG-supported synthesis of 3,5-disubstituted 1,2,4-triazoles
Wang, Jun-Ke,Zong, Ying-Xiao,Yue, Guo-Ren
, p. 1135 - 1136 (2005)
1,3-Dipolar cycloadditions between dieth...
The Synthesis of 1,3,5-Thiadiazinylium Salts
Shibuya, Isao
, p. 3369 - 3370 (1980)
1,3,5-Thiadiazinylium salts were synthes...
Liquid-phase traceless synthesis of 3,5-disubstituted 1,2,4-triazoles
Wang, Xi-Cun,Wang, Jun-Ke,Wu, Dong-Qing,Zong, Ying-Xiao
, p. 2595 - 2598 (2005)
A liquid-phase traceless route to 3,5-di...
Solid phase synthesis of 1,2,4-triazoles under microwave irradiation
Rostamizadeh, Shahnaz,Tajik, Hasan,Yazdanfarahi, Soheila
, p. 113 - 117 (2003)
1,2,4-Triazoles (3a-g) have been prepare...
1,2,4-Triazolato paddlewheel dibismuth complexes with very short Bi(ii)-Bi(ii) bonds: Bismuth(iii) oxidation of 1,2,4-triazolato anions into neutral: N -1,2,4-triazolyl radicals
He, Ru-Ru,Li, Li,Ma, Jian-Ping,Su, Ji-Hu,Zhang, Xiang,Zhao, Ming-Gang,Zheng, Wenjun
, p. 15190 - 15194 (2020)
Bismuth(iii) oxidation of 3,5-di-substit...
A base-catalyzed, direct synthesis of 3,5-disubstituted 1,2,4-triazoles from nitriles and hydrazides
Yeung, Kap-Sun,Farkas, Michelle E.,Kadow, John F.,Meanwell, Nicholas A.
, p. 3429 - 3432 (2005)
A convenient and efficient one step, bas...
Iodine Catalyzed Oxidative Coupling of Diaminoazines and Amines for the Synthesis of 3,5-Disubstituted-1,2,4-Triazoles
Beifuss, Uwe,Bharatam, Prasad V.,Chourasiya, Sumit S.,Kathuria, Deepika,Sahoo, Subash C.,Wani, Aabid A.
, p. 7659 - 7671 (2021/06/25)
A simple, convenient, transition metal-f...
TBHP/TBAI–Mediated simple and efficient synthesis of 3,5-disubstituted and 1,3,5-trisubstituted 1H-1,2,4-triazoles via oxidative decarbonylation of aromatic aldehydes and testing for antibacterial activities
Agisho, Habtamu Abebe,Esatu, Habdolo,Hairat, Suboot,Zaki, Mehvash
supporting information, (2020/05/19)
The author has developed a simple, effic...
A practical base mediated synthesis of 1,2,4-triazoles enabled by a deamination annulation strategy
Zhang, Chunyan,Liang, Zuyu,Jia, Xiaofei,Wang, Maorong,Zhang, Guoying,Hu, Mao-Lin
supporting information, p. 14215 - 14218 (2020/11/24)
A rapid and efficient base mediated synt...
Green aqueous synthesis and antimicrobial evaluation of 3,5-disubstituted 1,2,4-triazoles
Beyzaei, Hamid,Malekraisi, Farideh,Aryan, Reza,Ghasemi, Behzad
, p. 482 - 487 (2020/05/25)
[Figure not available: see fulltext.] An...
2039-06-7 Process route
-
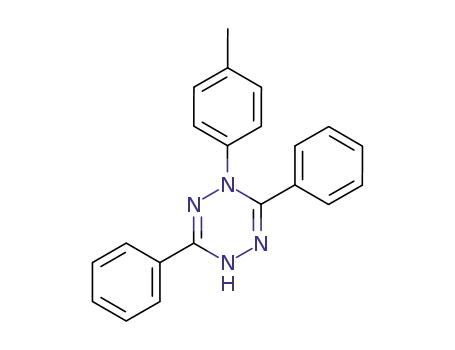
- 95309-27-6
1,4-dihydro-1-(4-methylphenyl)-3,6-diphenyl-s-tetrazine

-
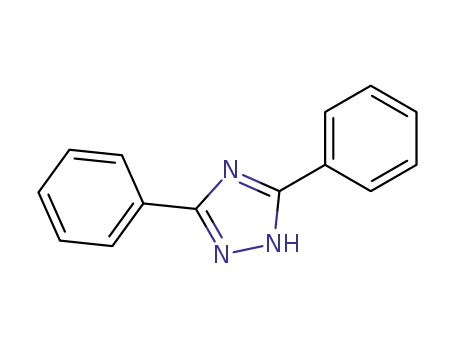
- 2039-06-7
3,5-diphenyl-1,2,4-triazole

-
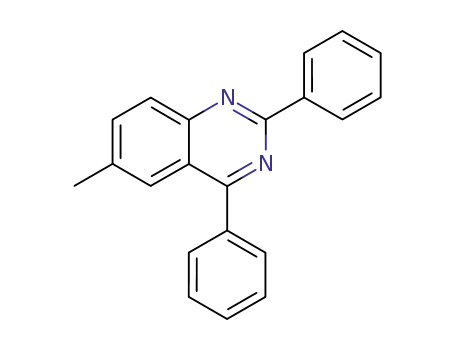
- 16107-83-8
6-methyl-2,4-diphenyl-quinazoline

-
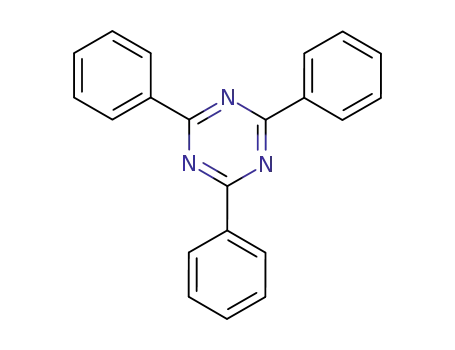
- 493-77-6
2,4,6-triphenyl-1,3,5-triazine

-
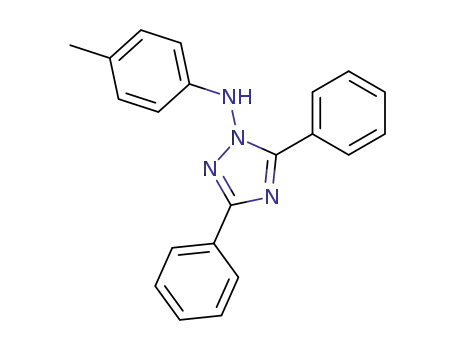
- 106910-90-1
3,5-diphenyl-1-(p-toluidino)-1H-1,2,4-triazole
| Conditions | Yield |
|---|---|
|
at 190 - 200 ℃; for 0.25h;
|
120 mg 57 mg |
-
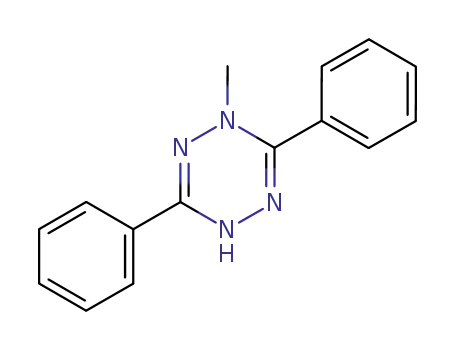
- 35401-80-0
1-Methyl-3,6-diphenyl-1,4-dihydro-1,2,4,5-tetrazin

-

- 2039-06-7
3,5-diphenyl-1,2,4-triazole

-
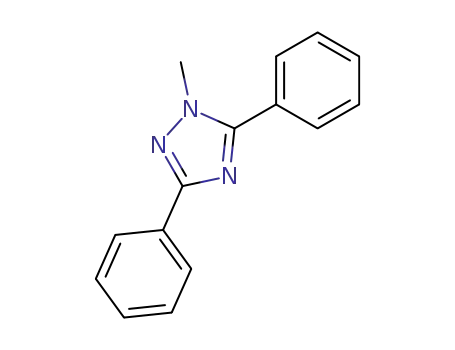
- 64017-21-6
3,5-diphenyl-1-methyl-1H-1,2,4-triazole

-

- 493-77-6
2,4,6-triphenyl-1,3,5-triazine

-
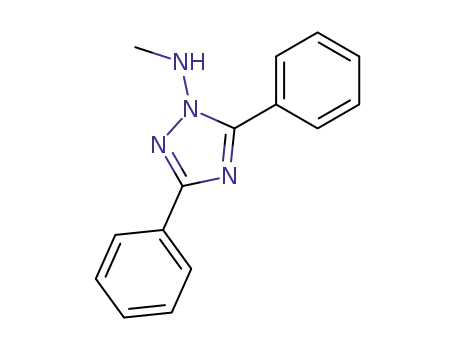
- 95309-33-4
1-methylamino-3,5-diphenyl-1,2,4-triazole
| Conditions | Yield |
|---|---|
|
for 0.25h; Further byproducts given; oil bath at 190 - 200 deg C;
|
0.154 g 0.098 g 0.050 g |
2039-06-7 Upstream products
-
729-44-2
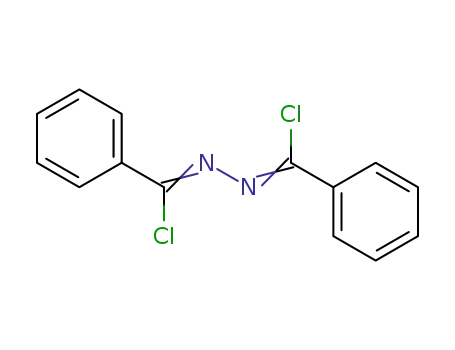
1,2-bis[chloro(phenyl)methylidene]hydrazone
-
93-89-0
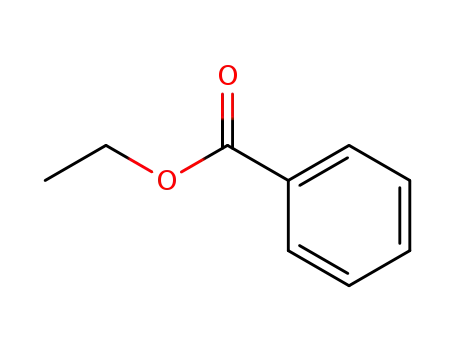
benzoic acid ethyl ester
-
614-28-8
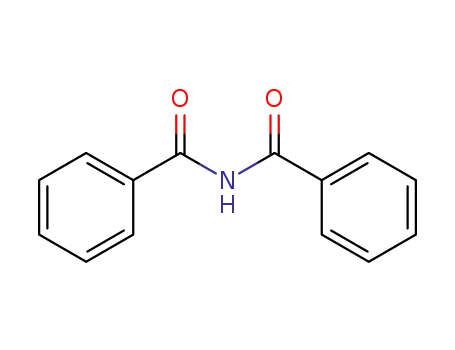
N-benzoylbenzamide
-
563-41-7
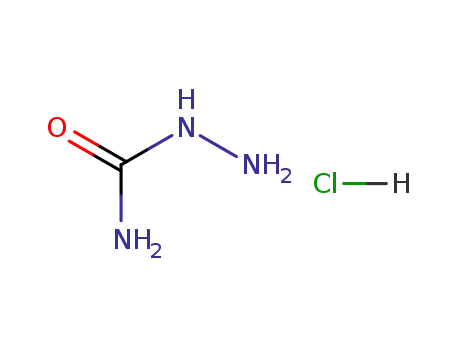
semicarbazide hydrochloride
2039-06-7 Downstream products
-
64017-21-6

3,5-diphenyl-1-methyl-1H-1,2,4-triazole
-
114505-92-9
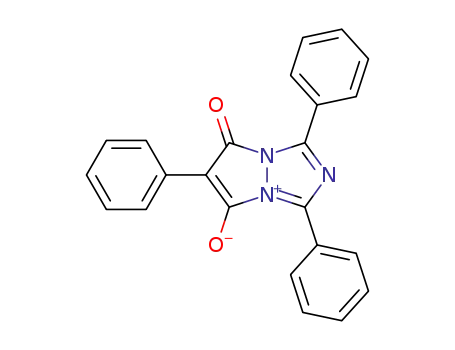
C23H15N3O2
-
80465-12-9
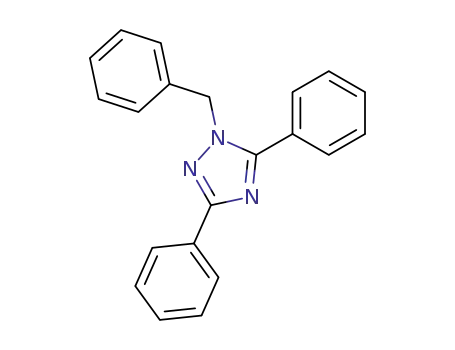
1-benzyl-3,5-diphenyl-1H-1,2,4-triazole
-
25855-18-9
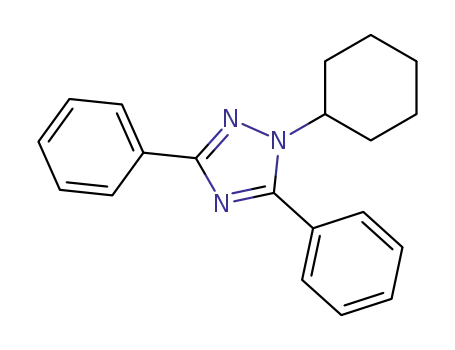
1-cyclohexyl-3,5-diphenyl-1H-1,2,4-triazole
Relevant Products
-
5-(2′-Hydroxy-3′-Naphthamide)-2-Benzimidazolone
CAS:26848-40-8
-
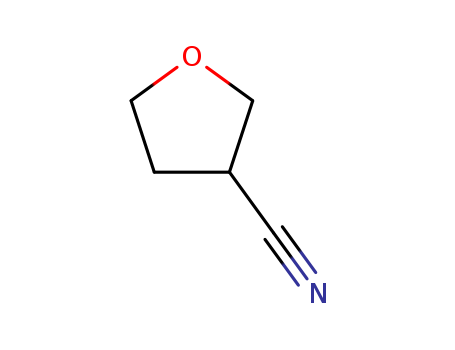
3-Cyanotetrahydrofuran
CAS:14631-44-8
-
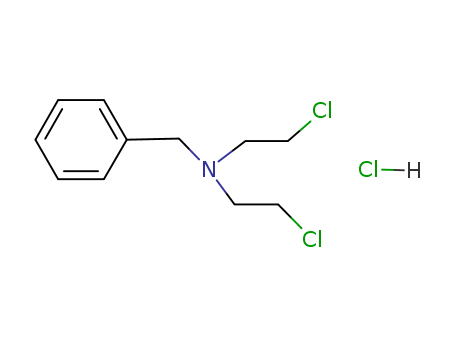
N-Benzyl-2,2'-dichloroethylamine Hydrochloride
CAS:10429-82-0