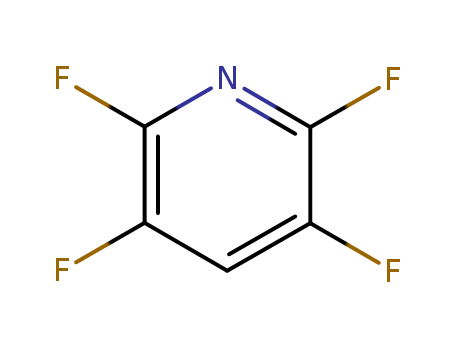
2875-18-5
- Product Name:2,3,5,6-Tetrafluoropyridine
- Molecular Formula:C5HF4N
- Purity:99%
- Molecular Weight:151.063
Product Details;
CasNo: 2875-18-5
Molecular Formula: C5HF4N
Appearance: clear colorless liquid
Factory Sells Best Quality 2,3,5,6-Tetrafluoropyridine 2875-18-5 with steady supply
- Molecular Formula:C5HF4N
- Molecular Weight:151.063
- Appearance/Colour:clear colorless liquid
- Vapor Pressure:42.2mmHg at 25°C
- Refractive Index:n20/D 1.4046(lit.)
- Boiling Point:106 °C at 760 mmHg
- PKA:-10.94±0.20(Predicted)
- Flash Point:30.6 °C
- PSA:12.89000
- Density:1.518 g/cm3
- LogP:1.63800
2,3,5,6-Tetrafluoropyridine(Cas 2875-18-5) Usage
|
Synthesis |
Catalytic reduction of aryl chlorides, bromides and iodides is well documented, however, aryl fluorides do not readily react under these conditions, owing to the strong C-F bond and the high activation barrier to bond breaking. Work in our laboratories showed that at high temperatures, pentafluoropyridine was converted to 2,3,5,6- tetrafluoropyridine using a palladium catalyst. |
|
Preparation |
An autoclave sprayed with PTFE was charged with pentafluoropyridine (20.0 g, ?118 mmol), HBr (32.0 g, 400 mmol) and sulpholane (40 cm3) and heated at 200 ℃ for ?48 h. The mixture was added to water and extracted into ether. The ether solution was ?shown to contain pentafluoropyridine (79%) and 2,3,5,6-tetrafluoropyridine (21%) by ?comparison of their GCMS and fluorine nmr spectra with authentic samples. |
InChI:InChI=1/C5H8F2O2/c1-3-9-4(8)5(2,6)7/h3H2,1-2H3
2875-18-5 Relevant articles
Mechanistic study of Ru-NHC-catalyzed hydrodefluorination of fluoropyridines: The influence of the NHC on the regioselectivity of C-F activation and chemoselectivity of C-F versus C-H bond cleavage
McKay, David,Riddlestone, Ian M.,Macgregor, Stuart A.,Mahon, Mary F.,Whittlesey, Michael K.
, p. 776 - 787 (2015)
We describe a combined experimental and ...
Dual C-F, C-H Functionalization via Photocatalysis: Access to Multifluorinated Biaryls
Senaweera, Sameera,Weaver, Jimmie D.
, p. 2520 - 2523 (2016)
Multifluorinated biaryls are challenging...
Dual Photoredox-/Palladium-Catalyzed Cross-Electrophile Couplings of Polyfluoroarenes with Aryl Halides and Triflates
Qin, Jian,Zhu, Shengqing,Chu, Lingling
supporting information, p. 2246 - 2252 (2021/04/02)
A visible-light photoredox-/Pd-catalyzed...
Photoredox/Nickel Dual-Catalyzed Reductive Cross Coupling of Aryl Halides Using an Organic Reducing Agent
Bülow, Raoul F.,Dewanji, Abhishek,Rueping, Magnus
supporting information, p. 1611 - 1617 (2020/03/13)
A successful protocol for the reductive ...
Dihydridoboranes: Selective Reagents for Hydroboration and Hydrodefluorination
Phillips, Nicholas A.,O'hanlon, James,Hooper, Thomas N.,White, Andrew J. P.,Crimmin, Mark R.
supporting information, p. 7289 - 7293 (2019/10/08)
The preparation of a new series of dihyd...
Hydrodefluorination of fluoroaromatics by isopropyl alcohol catalyzed by a ruthenium NHC complex. An unusual role of the carbene ligand
Mai, Van Hung,Nikonov, Georgii I.
, p. 7956 - 7961 (2018/05/23)
The NHC (NHC = N-heterocyclic carbene) c...
2875-18-5 Process route
-
-
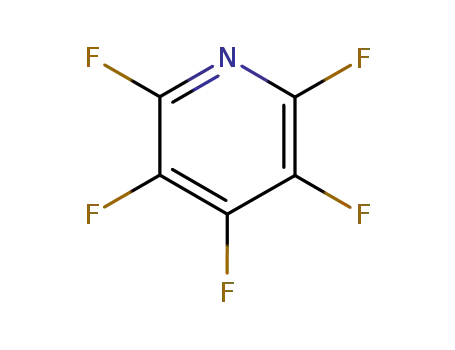
700-16-3
Pentafluoropyridine

-
-
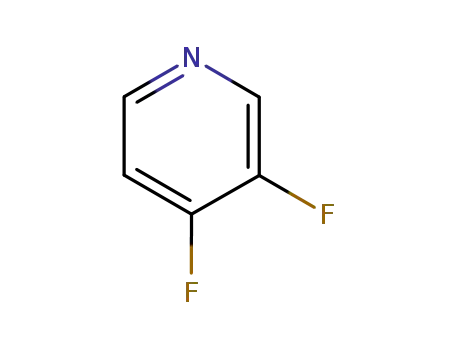
82878-63-5
3,4-difluoropyridine

-
-
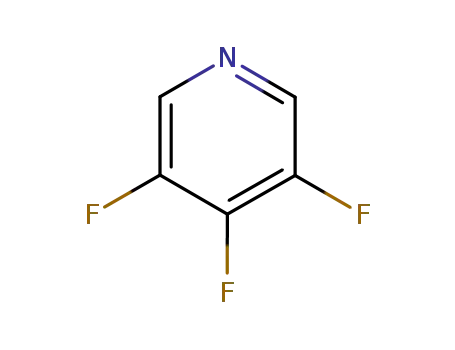
67815-54-7
3,4,5-trifluoropyridine

-
-
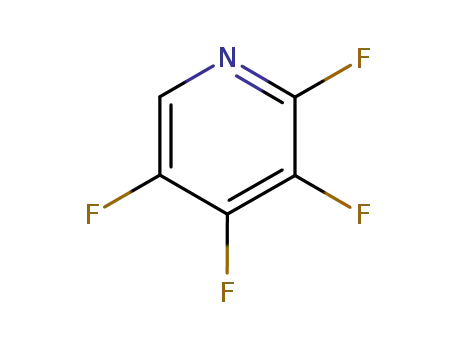
3512-16-1
2,3,4,5-tetrafluoropyridine

-
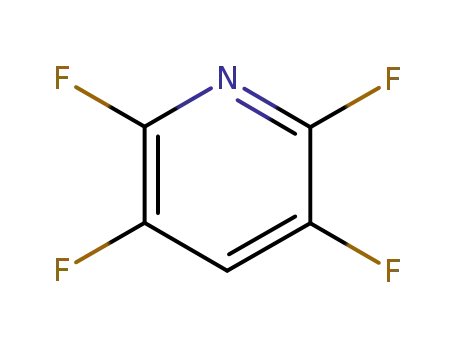
-
2875-18-5
2,3,5,6-tetrafluoropyridine

-
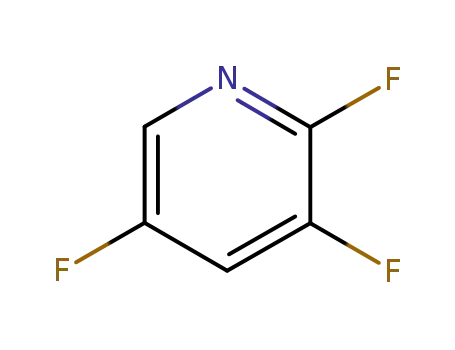
-
76469-41-5
2,3,5‐trifluoropyridine
| Conditions | Yield |
|---|---|
|
With
triethylsilane; α,α,α-trifluorotoluene; C64H68N2OP2Ru;
In
tetrahydrofuran;
at 69.84 ℃;
for 28h;
Time;
stereoselective reaction;
Schlenk technique;
Glovebox;
|
42 %Spectr. 22 %Spectr. 21 %Spectr. 7 %Spectr. 3 %Spectr. |
|
With
triethylsilane; α,α,α-trifluorotoluene; C64H68N2OP2Ru;
In
tetrahydrofuran;
at 89.84 ℃;
for 28h;
Time;
Temperature;
stereoselective reaction;
Schlenk technique;
Glovebox;
|
41 %Spectr. 26 %Spectr. 20 %Spectr. 8 %Spectr. 2 %Spectr. |
-
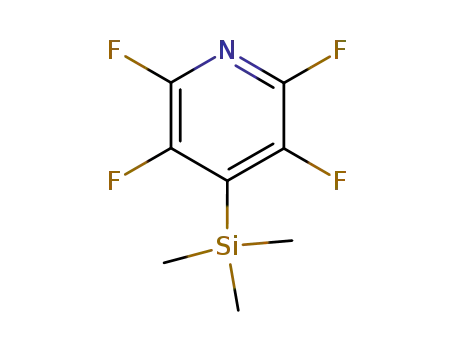
-
16297-29-3
2,3,5,6-tetrafluoropyridin-4-yltrimethylsilan

-

-
2875-18-5
2,3,5,6-tetrafluoropyridine

-

-
700-16-3
Pentafluoropyridine

-
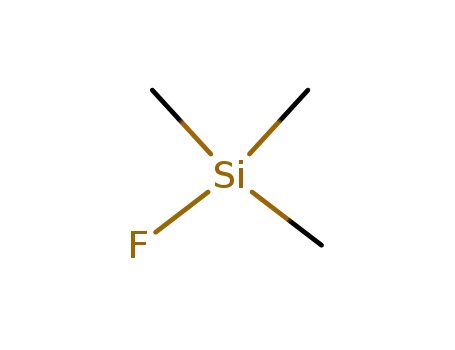
-
420-56-4
trimethylsilyl fluoride

-
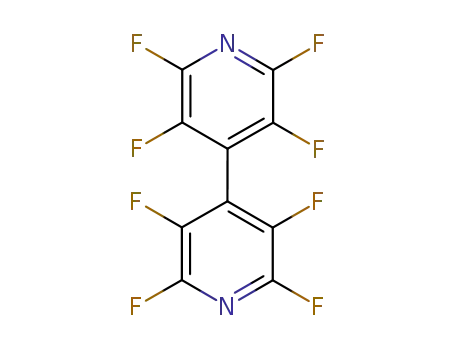
-
3511-91-9
octafluoro dipyridyl-(4,4')
| Conditions | Yield |
|---|---|
|
With
xenon difluoride; tetramethylammonium fluoride;
In
dichloromethane;
at -60 ℃;
for 0.166667h;
Title compound not separated from byproducts.;
|
2875-18-5 Upstream products
-
16297-29-3
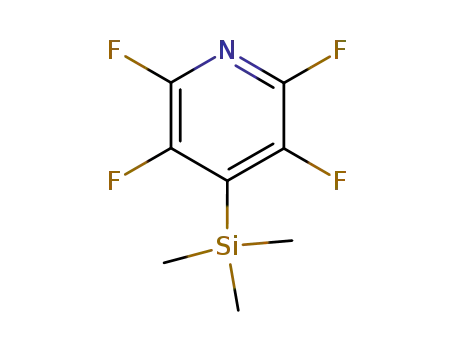
2,3,5,6-tetrafluoropyridin-4-yltrimethylsilan
-
71-43-2
benzene
-
75-16-1
methylmagnesium bromide
-
16297-07-7
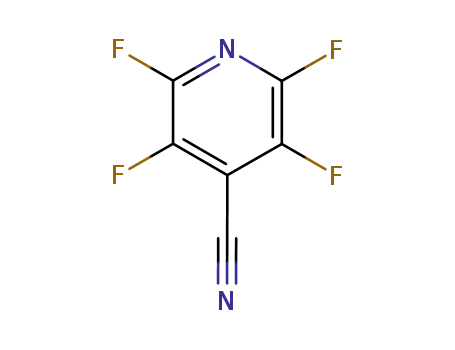
2,3,5,6-tetrafluoro-4-pyridinecarbonitrile
2875-18-5 Downstream products
-
3534-50-7
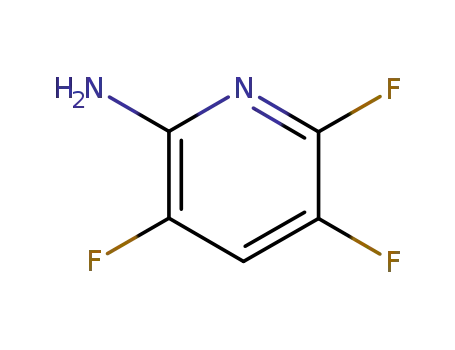
2-Amino-3,5,6-trifluoropyridine
-
379-50-0
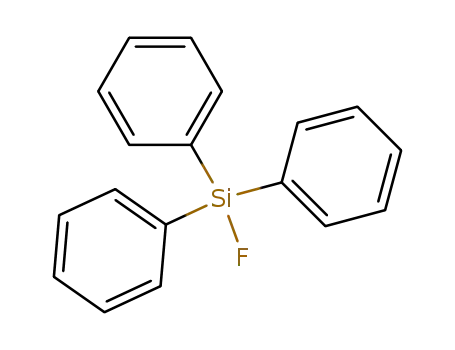
triphenylsilyl fluoride
-
789-25-3
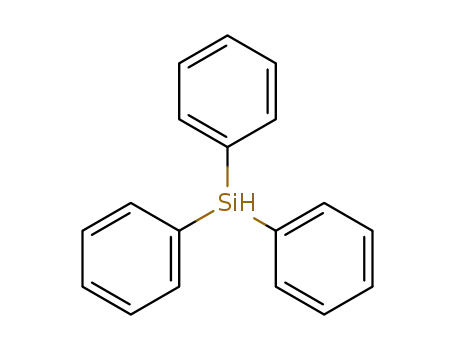
HSiPh3
Relevant Products
-
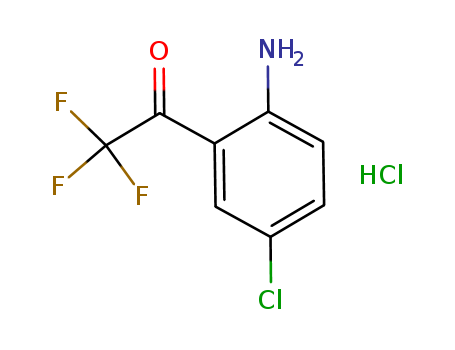
4-Chloro-2-(Trifluoroacetyl)aniline Hydrochloride
CAS:173676-59-0
-
Cisplatin Acid
CAS:82009-34-5