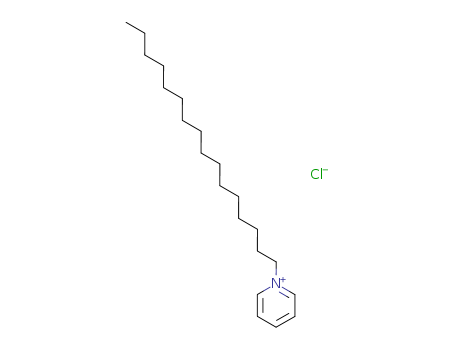
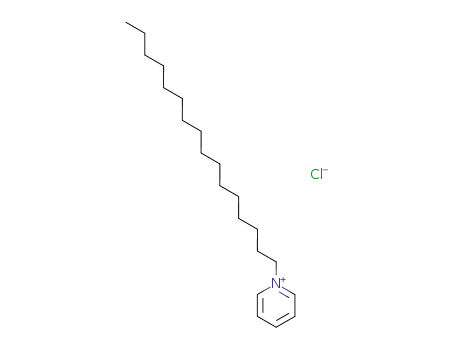
123-03-5
- Product Name:Cetylpyridinium chloride
- Molecular Formula:C21H38ClN
- Purity:99%
- Molecular Weight:339.992
Product Details;
CasNo: 123-03-5
Molecular Formula: C21H38ClN
Appearance: white powder or crystals
Factory Sells Best Quality Cetylpyridinium chloride 123-03-5 with GMP standards
- Molecular Formula:C21H38ClN
- Molecular Weight:339.992
- Appearance/Colour:white powder or crystals
- Vapor Pressure:0Pa at 25℃
- Melting Point:77 °C
- Refractive Index:1.6000 (estimate)
- Boiling Point:496.25°C (rough estimate)
- PSA:3.88000
- Density:0.9362 (rough estimate)
- LogP:3.45940
Cetylpyridinium chloride(Cas 123-03-5) Usage
|
Production Methods |
Cetylpyridinium chloride is prepared from cetyl chloride by treatment with pyridine. |
|
Flammability and Explosibility |
Nonflammable |
|
Pharmaceutical Applications |
Cetylpyridinium chloride is a quaternary ammonium cationic surfactant, used in pharmaceutical and cosmetic formulations as an antimicrobial preservative. It is used therapeutically as an antiseptic agent; used alone or in combination with other drugs for oral and throat care; used in nonparenteral formulations licensed in the UK; and used in oral and inhalation preparations at concentrations of 0.02–1.5 mg. Mouthwashes containing cetylpyridinium chloride have been shown to inhibit plaque formation,(1–3) although efficacy is variable owing to limited published data. |
|
Safety Profile |
Poison by ingestion, intraperitoneal, subcutaneous, and intravenous routes. Moderately toxic by skin contact. A skin and eye irritant. When E heated to decomposiuon it emits very toxic fumes of NOx and Cl-. |
|
Safety |
Cetylpyridinium chloride is used widely in mouthwashes as a bactericidal antiseptic. It is generally regarded as a relatively nontoxic material when used at a concentration of 0.05% w/v, although minor side effects such as mild burning sensations on the tongue have been reported. At higher concentrations, cetylpyridinium chloride may damage the mucous membranes in the mouth. It is harmful when ingested or inhaled. It can cause eye irritation, and is irritant to the respiratory system and the skin. LD50 (rat, IP): 0.006 g/kg LD50 (rat, IV): 0.03 g/kg LD50 (rat, oral): 0.2 g/kg LD50 (rat, SC): 0.25 g/kg LD50 (mouse, IP): 0.01 g/kg LD50 (mouse, oral): 0.108 g/kg LD50 (rabbit, oral): 0.4 g/kg LD50 (rabbit, IV): 0.036 g/kg |
|
storage |
Cetylpyridinium chloride is stable under normal conditions. It should be stored in well-closed containers. |
|
Incompatibilities |
Incompatible with strong oxidizing agents and bases. It is also incompatible with methylcellulose. Magnesium stearate suspensions in cetylpyridinium chloride have been shown to significantly reduce its antimicrobial activity. This is due to the absorption of cetylpyridinium chloride on magnesium stearate. The cetylpyridinium chloride ion also interacts with gelatin, resulting in reduced bioavailability. |
|
Regulatory Status |
Included in nonparenteral formulations licensed in the UK. Included in the FDA Inactive Ingredients Database, for use in inhalation and oral preparations. Reported in the EPA TSCA Inventory. It is not approved for use in Japan. Included in the Canadian List of Acceptable Non-medicinal Ingredients. |
|
Definition |
ChEBI: A pyridinium salt that has N-hexadecylpyridinium as the cation and chloride as the anion. It has antiseptic properties and is used in solutions or lozenges for the treatment of minor infections of the mouth and throat. |
|
Brand name |
Ceepryn (Marion Merrell Dow); Cepacol (Marion Merrell Dow). |
InChI:InChI=1/C21H38N.2ClH/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-16-19-22-20-17-15-18-21-22;;/h15,17-18,20-21H,2-14,16,19H2,1H3;2*1H/q+1;;/p-2
123-03-5 Relevant articles
Surfactant Aggregation Number and Polydispersity of SDS + 1-Pentanol Mixed Micelles in Brine Determined by Time-Resolved Fluorescence Quenching
Lang, Jacques
, p. 3734 - 3739 (1990)
Size and polydispersity of sodium dodecy...
AN IMPROVED PROCESS FOR PREPARATION OF QUATERNARY PYRIDINIUM SALTS
-
Page/Page column 10; 11, (2019/08/08)
The present invention discloses a proces...
AN IMPROVED PROCESS FOR THE PRODUCTION OF QUATERNARY PYRIDINIUM SALTS
-
Page/Page column 8, (2016/06/28)
Disclosed herein is cost effective and e...
STABLE COMPOSITIONS OF THIABENDAZOLE AND IODINE-CONTAINING FUNGICIDES
-
, (2015/02/25)
The present invention relates to stable ...
FUNGICIDAL PENFLUFEN MIXTURES
-
, (2014/04/03)
The invention relates to mixtures compri...
123-03-5 Process route
-
4-(4-Dimethylamino-phenylazo)-benzenesulfonate1-hexadecyl-pyridinium;
-
4-(4-Dimethylamino-phenylazo)-benzenesulfonate1-hexadecyl-pyridinium;

-
- 123-03-5
cetylpyridinium chloride

-
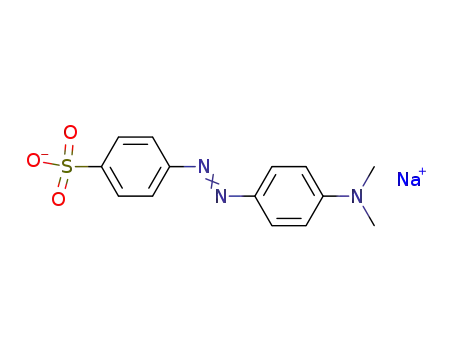
- 547-58-0
methyl orange
| Conditions | Yield |
|---|---|
|
With sodium chloride; at 19.9 ℃; Equilibrium constant; var. temps.;
|
-
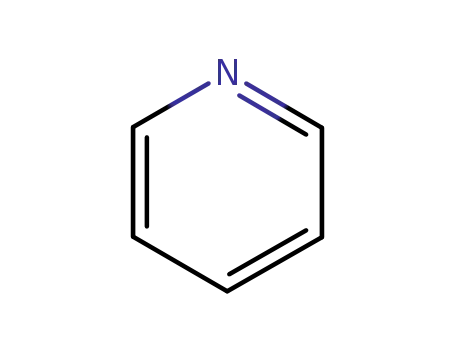
- 110-86-1
pyridine

-

- 4860-03-1
1-Chlorohexadecan

-

- 123-03-5
cetylpyridinium chloride
| Conditions | Yield |
|---|---|
|
|
|
|
In ethanol; at 135 ℃; for 4h;
|
|
|
at 100 ℃; for 168h;
|
|
|
at 90 - 100 ℃; Reagent/catalyst; Large scale;
|
12.6 kg |
|
In water; at 95 - 102 ℃; for 30h; Large scale;
|
1200 g |
123-03-5 Upstream products
-
110-86-1

pyridine
-
4860-03-1

1-Chlorohexadecan
-
58527-29-0
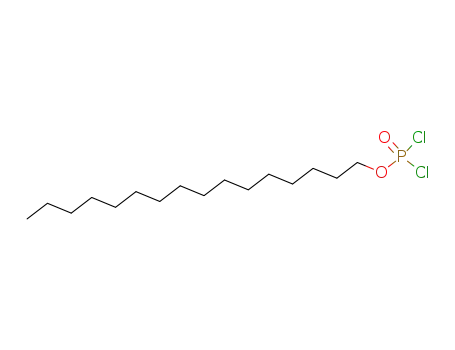
hexadecyl dichlorophosphinate
-
112-82-3

hexadecanyl bromide
123-03-5 Downstream products
-
129-00-0
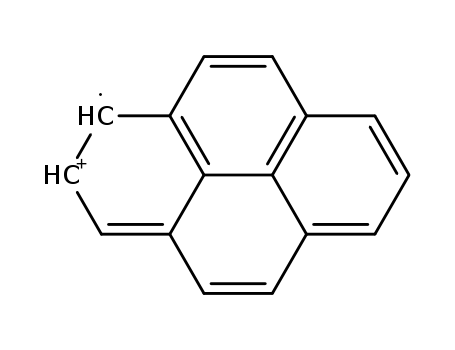
radical cation of pyrene
-
110-86-1

pyridine
-
629-73-2
1-Hexadecene
-
744221-35-0
cetylpyridinium salt of diclofenac
Relevant Products
-
Cetyltrimethyl Ammonium Chloride
CAS:112-02-7
-
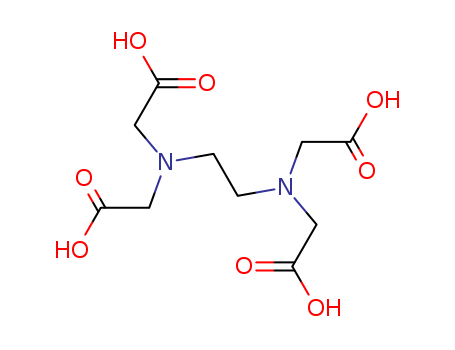
Ethylenediaminetetraacetic Acid
CAS:60-00-4
-
Benzalkonium Chloride
CAS:63449-41-2