112-72-1
- Product Name:C14 Alcohol
- Molecular Formula:C14H30O
- Purity:99%
- Molecular Weight:214.392
Product Details;
CasNo: 112-72-1
Molecular Formula: C14H30O
Appearance: white low melting solid or flakes
Buy reliable Quality C14 Alcohol 112-72-1 raw material with Honest Price
- Molecular Formula:C14H30O
- Molecular Weight:214.392
- Appearance/Colour:white low melting solid or flakes
- Vapor Pressure:<1 hPa (20 °C)
- Melting Point:35-39 °C(lit.)
- Refractive Index:1.4454
- Boiling Point:263.2 °C at 760 mmHg
- PKA:15.20±0.10(Predicted)
- Flash Point:125.9 °C
- PSA:20.23000
- Density:0.833 g/cm3
- LogP:4.67980
1-Tetradecanol(Cas 112-72-1) Usage
|
Production Methods |
Myristyl alcohol is found in spermaceti wax and sperm oil, and may be synthesized by sodium reduction of fatty acid esters or the reduction of fatty acids by lithium aluminum hydride. It can also be formed from acetaldehyde and dimethylamine. |
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 35, p. 1210, 1970 DOI: 10.1021/jo00829a089 |
|
Air & Water Reactions |
Insoluble in water. |
|
Reactivity Profile |
1-Tetradecanol is an alcohol. Flammable and/or toxic gases are generated by the combination of alcohols with alkali metals, nitrides, and strong reducing agents. They react with oxoacids and carboxylic acids to form esters plus water. Oxidizing agents convert them to aldehydes or ketones. Alcohols exhibit both weak acid and weak base behavior. They may initiate the polymerization of isocyanates and epoxides. |
|
Health Hazard |
Low toxicity. Overexposure causes some central nervous system depression. Prolonged skin contact causes skin irritation. |
|
Flammability and Explosibility |
Notclassified |
|
Pharmaceutical Applications |
Myristyl alcohol is used in oral, parenteral, and topical pharmaceutical formulations. It has been evaluated as a penetration enhancer in melatonin transdermal patches in rats. Myristyl alcohol has also been tested as a bilayer stabilizer in niosome formulations containing ketorolac tromethamine,and zidovudine.Niosomes containing myristyl alcohol showed a considerably slower release rate of ketorolac tromethamine than those containing cholesterol.This was also observed with the zidovudine formulation. |
|
Safety |
Myristyl alcohol is used in oral parenteral, and topical pharmaceutical formulations. The pure form of myristyl alcohol is mildly toxic by ingestion and may be carcinogenic; experimental tumorigenic data are available.It is also a human skin irritant. In animal studies of the skin permeation enhancement effect of saturated fatty alcohols, myristyl alcohol exhibited a lower effect when compared with decanol, undecanol, or lauryl alcohol but caused greater skin irritation.A study investigating contact sensitization to myristyl alcohol revealed that patch testing of myristyl alcohol 10% petrolatum should not be carried out owing to observed irritant effects; thus the use of a lower concentration of myristyl alcohol for such tests (5% petrolatum) was recommended.Myristyl alcohol has been associated with some reports of contact allergy.(8,9)A moderate-to-severe erythema and moderate edema are seen when 75 mg is applied to human skin intermittently in three doses over 72 hours. LD50(rabbit, skin): 7.1 g/kg LD50(rat, oral): 33.0 g/kg |
|
storage |
The bulk material should be stored in a well-closed container in a cool, dry place. |
|
Purification Methods |
Crystallise the alcohol from aqueous EtOH. It has also been purified by zone melting. [Beilstein 1 IV 1864.] |
|
Incompatibilities |
Myristyl alcohol is combustible when exposed to heat or flame. It can react with oxidizing materials. When heated to decomposition, it emits acrid smoke and irritating fumes. |
|
Regulatory Status |
Included in the FDA Inactive Ingredients Database (oral tablet: sustained-release; and topical formulations: cream, lotion, suspension). Included in nonparenteral (topical cream) formulations licensed in the UK. |
|
Definition |
ChEBI: 1-Tetradecanol is a long-chain fatty alcohol that is tetradecane in which one of the terminal methyl hydrogens is replaced by a hydroxy group It is a long-chain primary fatty alcohol, a fatty alcohol 14:0 and a primary alcohol. |
|
General Description |
Colorless thick liquid (heated) with a faint alcohol odor. Solidifies and floats on water. |
InChI:InChI=1/2C14H30O/c2*1-2-3-4-5-6-7-8-9-10-11-12-13-14-15/h2*15H,2-14H2,1H3
112-72-1 Relevant articles
Calcium borohydride: A reagent for facile conversion of carboxylic esters to alcohols and aldehydes
Narasimhan,Ganeshwar Prasad,Madhavan
, p. 1689 - 1697 (1995)
Calcium borohydride reduces both aliphat...
A simple alkene-catalyzed reduction of aromatic esters to alcohols by zinc borohydride
Narasimhan,Madhavan,Ganeshwar Prasad
, p. 385 - 390 (1997)
Reactivity of Zn(BH4)2 was modified by a...
Fatty alcohol synthesis from fatty acids at mild temperature by subsequent enzymatic esterification and metal-catalyzed hydrogenation
Betke, Tobias,Gr?ger, Harald,Kleber, Joscha,Liese, Andreas,Schlipk?ter, Kim E.
, p. 7862 - 7867 (2020)
Fatty alcohols are important products in...
OXO ACIDS AND BRANCHED FATTY ACID ESTERS FROM RHIZOMES OF COSTUS SPECIOSUS
Gupta, Madan M.,Verma, Ram K.,Akhila, Anand
, p. 1899 - 1902 (1986)
Key Word Index - Costus speciosus; Costa...
Synthesis and surface-active properties of novel cleavable gemini surfactants
Gilbert, Elangeni Ana,Guastavino, Javier Fernando,Murguía, Marcelo César
, p. 27 - 35 (2021/09/14)
A novel series of quaternary ammonium ge...
Borane evolution and its application to organic synthesis using the phase-vanishing method
Soga, Nene,Yoshiki, Tomo,Sato, Aoi,Kawamoto, Takuji,Ryu, Ilhyong,Matsubara, Hiroshi
supporting information, (2021/03/26)
Although borane is a useful reagent, it ...
Redox-active ligand based Mn(i)-catalyst for hydrosilylative ester reduction
Chakraborty, Soumi,Das, Arpan,Mandal, Swadhin K.
supporting information, p. 12671 - 12674 (2021/12/04)
Herein a Mn(i) catalyst bearing a redox-...
Enantiomeric synthesis of natural alkylglycerols and their antibacterial and antibiofilm activities
Fernández Montoya, Deicy J.,Contreras Jordan, Luis A.,Moreno-Murillo, Bárbara,Silva-Gómez, Edelberto,Mayorga-Wandurraga, Humberto
supporting information, p. 2544 - 2550 (2019/11/13)
Alkylglycerols (AKGs) are bioactive natu...
112-72-1 Process route
-
-
112-41-4,25067-08-7,62132-67-6
1-dodecene

-
-
1120-36-1
1-tetradecene

-
-
872-05-9,17438-89-0,25189-70-2
1-Decene

-
-
821-95-4
1-undecene

-
-
2437-56-1
1-tridecene

-
-
13360-61-7
1-pentadecene

-
-
201230-82-2
carbon monoxide

-
-
112-42-5
undecyl alcohol

-
-
629-76-5
pentadecanol

-
-
112-53-8
1-dodecyl alcohol

-
-
112-70-9
tridecan-1-ol

-
-
112-72-1
1-Tetradecanol

-

-
36653-82-4,124-29-8
1-Hexadecanol
| Conditions | Yield |
|---|---|
|
With
hydrogen;
tributylphosphine; cobalt(II) decanoate;
In
n-heptane;
at 170 ℃;
for 48h;
under 63756.4 Torr;
Conversion of starting material;
|
-
-
21129-09-9
tetradecane-1,2-diol

-
-
629-59-4
tetradecane

-

-
112-72-1
1-Tetradecanol
| Conditions | Yield |
|---|---|
|
With
C12H36Cl2O4RuS4; hydrogen;
In
benzene;
at 200 ℃;
for 96h;
under 21281.4 Torr;
|
9 %Chromat. 36 %Chromat. |
112-72-1 Upstream products
-
1120-36-1

1-tetradecene
-
62936-14-5
(E)-5-tetradecenol
-
124-25-4

myristylaldehyde
-
124-10-7
methyl myristoate
112-72-1 Downstream products
-
51874-67-0
phthalic acid monotetradecyl ester
-
2915-60-8

phthalic acid ditetradecyl ester
-
5451-63-8
tetradecyl formate
-
26719-47-1
Di-n-tetradecyl sebacate
Relevant Products
-
5-(2′-Hydroxy-3′-Naphthamide)-2-Benzimidazolone
CAS:26848-40-8
-
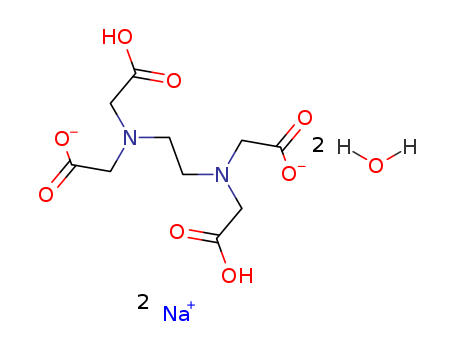
Disodium Ethylenediaminetetraacetic Acid
CAS:6381-92-6
-
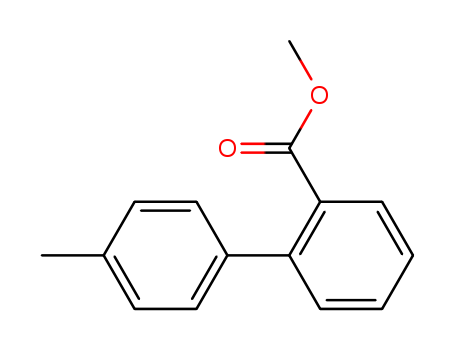
4'-Methylbiphenyl-2-Carboxylic Acid Methyl Ester
CAS:114772-34-8