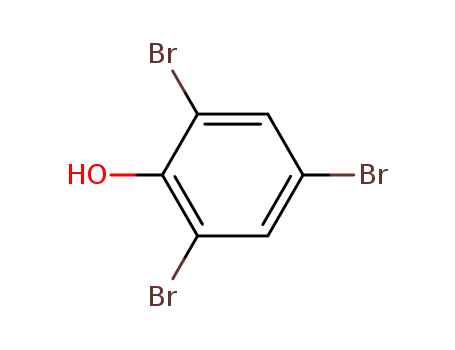
118-79-6
- Product Name:Tribromophenol (TBP)
- Molecular Formula:C6H3Br3O
- Purity:99%
- Molecular Weight:330.801
Product Details;
CasNo: 118-79-6
Molecular Formula: C6H3Br3O
Appearance: Soft, long, white crystals with a bromine odor.
Purity 99% Min Tribromophenol (TBP) 118-79-6 Spot Supply with Safe Transportation
- Molecular Formula:C6H3Br3O
- Molecular Weight:330.801
- Appearance/Colour:Soft, long, white crystals with a bromine odor.
- Vapor Pressure:0.0015mmHg at 25°C
- Melting Point:90-94 °C(lit.)
- Refractive Index:1.674
- Boiling Point:286.8 °C at 760 mmHg
- PKA:6.34±0.23(Predicted)
- Flash Point:109.7 °C
- PSA:20.23000
- Density:2.55 g/cm3
- LogP:3.67970
2,4,6-Tribromophenol(Cas 118-79-6) Usage
|
Definition |
ChEBI: 2,4,6-tribromophenol is a bromophenol that is phenol in which the hydrogens at positions 2, 4 and 6 have been replaced by bromines. It is commonly used as a fungicide and in the preparation of flame retardants. It has a role as an environmental contaminant, a fungicide and a marine metabolite. |
|
Production Methods |
2,4,6-Tribromophenol is produced by the controlled bromination of phenol. |
|
General Description |
Soft, long, white crystals with a bromine odor. |
|
Air & Water Reactions |
Slightly soluble in water. |
|
Reactivity Profile |
2,4,6-Tribromophenol can react with oxidizing materials . |
|
Hazard |
By ingestion, inhalation, skin absorption. Strong skin irritant. |
|
Fire Hazard |
Data is not available for 2,4,6-Tribromophenol. 2,4,6-Tribromophenol is probably non-flammable. |
|
Flammability and Explosibility |
Nonflammable |
|
Purification Methods |
Crystallise the phenol from EtOH or pet ether. Dry it under vacuum over P2O5 at room temperature. [Beilstein 6 IV 1067.] |
InChI:InChI=1/C6H3Br3O/c7-3-1-4(8)6(10)5(9)2-3/h1-2,10H
118-79-6 Relevant articles
Bromination of Phenols by Use of Benzyltrimethylammonium Tribromide
Kajigaeshi, Shoji,Kakinami, Takaaki,Tokiyama, Hajime,Hirakawa, Takahiro,Okamoto, Tsuyoshi
, p. 627 - 630 (1987)
The reaction of phenols with benzyltrime...
-
Lewis
, p. 1003 (1902)
-
Reaction mechanism for the cyclization of 3-[γ,γ-dimethylallyl] coumaric acid methyl ester in dimethyl sulfoxide (DMSO)
Borkowski,Ardanaz,Rossomando,Tonn
, p. 612 - 613 (2000)
-
-
Day
, p. 646,648, 649 (1930)
-
-
Kemp
, p. 202 (1971)
-
Kinetics and mechanism of oxidation of aspirin by bromamine-T, N-bromosuccinimide, and N-bromophthalimide
Ramachandrappa,Puttaswamy,Mayanna,Made Gowda
, p. 407 - 414 (1998)
The kinetics of the oxidation of aspirin...
Electrophilic bromination in flow: A safe and sustainable alternative to the use of molecular bromine in batch
Van Kerrebroeck, Reinout,Naert, Pieter,Heugebaert, Thomas S.A.,D’hooghe, Matthias,Stevens, Christian V.
, (2019)
Bromination reactions are crucial in tod...
-
Werner
, p. 373 (1885)
-
-
Bamberger,Kraus
, p. 265 (1922)
-
Enthalpies of formation of 2,4,6-tribromophenol and of 2,4,6-tribromoaniline
Allot, Philip H.,Finch, Arthur,Pilcher, Geoffrey,Nunez, Lisardo,Barral, Luis
, p. 771 - 780 (1987)
The standard (p0 = 101.325 kPa) molar en...
-
Tsubota,M. et al.
, p. 1252 - 1253 (1972)
-
Studies directed toward total synthesis of rhodocomatulins: A regioselective synthesis of brominated hydroxyanthraquinones by anionic annulations
Sk, Md Raja,Chakraborty, Soumen,Mal, Dipakranjan
, p. 309 - 317 (2018)
In this work, brominated hydroxyanthraqu...
Benzylic oxidation by the GIF(IV) system
Barton,Halley,Ozbalik,Mehl
, p. 6615 - 6618 (1989)
Oxidation of ethylbenzene, diphenylmetha...
Oxidovanadium (V and IV) complexes incorporating coumarin based O^N^O ligand: Synthesis, structure and catalytic activities
Majumder, Mitali,Krishna Rajak, Kajal
, (2020)
The tridentate ligand H2L1, [(E)-7-Hydro...
-
Lauer,Langkammerer
, p. 1628 (1934)
-
-
Orton,Coates,Burdett
, p. 51 (1907)
-
-
Lloyd
, p. 8 (1905)
-
A scalable and green one-minute synthesis of substituted phenols
Elumalai, Vijayaragavan,Hansen, J?rn H.
, p. 40582 - 40587 (2020/11/18)
A mild, green and highly efficient proto...
A convenient and efficient H2SO4-promoted regioselective monobromination of phenol derivatives using N-bromosuccinimide
Wu, Yong-Qi,Lu, Hai-Jia,Zhao, Wen-Ting,Zhao, Hong-Yi,Lin, Zi-Yun,Zhang, Dong-Feng,Huang, Hai-Hong
supporting information, p. 813 - 822 (2020/02/15)
A convenient, rapid H2SO4-promoted regio...
118-79-6 Process route
-
-
946-80-5
(benzyloxy)benzene

-
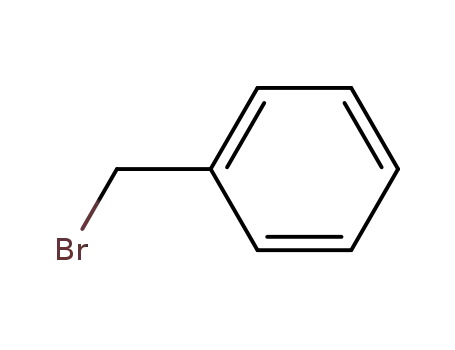
-
100-39-0
benzyl bromide

-
-
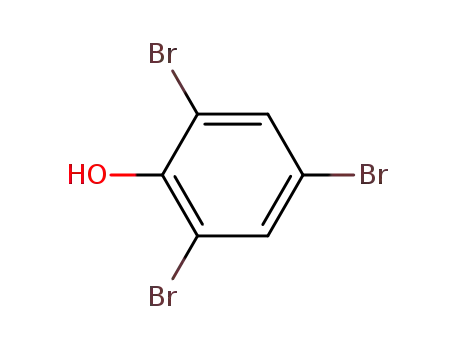
118-79-6
2,4,6-tribromophenol
| Conditions | Yield |
|---|---|
|
With
bromine;
|
-
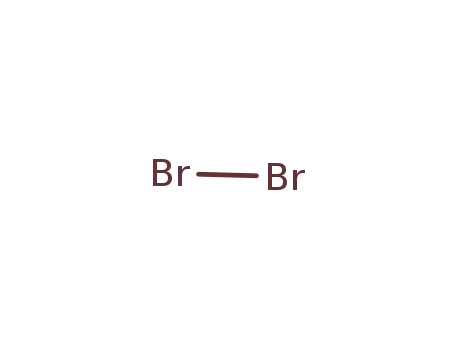
-
7726-95-6
bromine

-
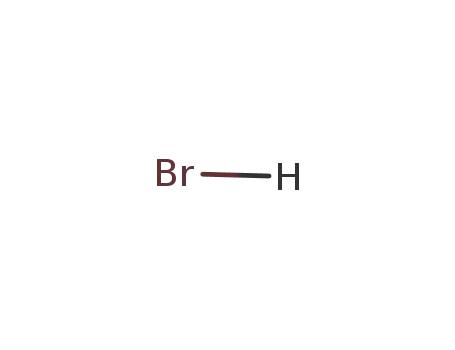
-
10035-10-6,12258-64-9
hydrogen bromide

-

-
118-79-6
2,4,6-tribromophenol
| Conditions | Yield |
|---|---|
|
With
chlorine; phenol;
In
water;
|
118-79-6 Upstream products
-
67-56-1
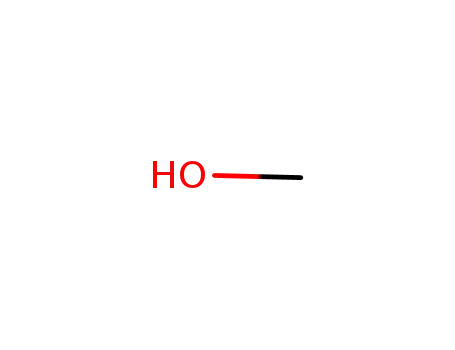
methanol
-
56-23-5
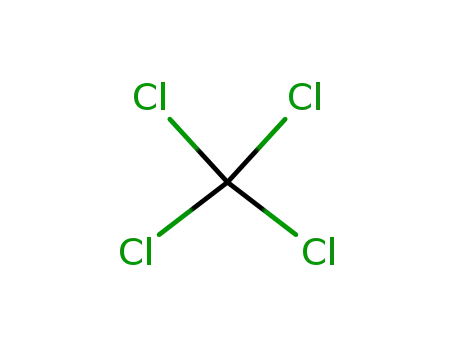
tetrachloromethane
-
20244-61-5
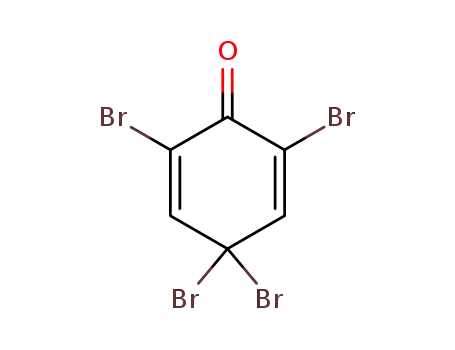
2,4,4,6-Tetrabromo-2,5-cyclohexadien-1-one
-
64-18-6
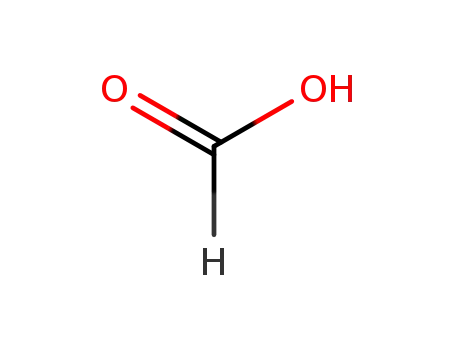
formic acid
118-79-6 Downstream products
-
23976-66-1
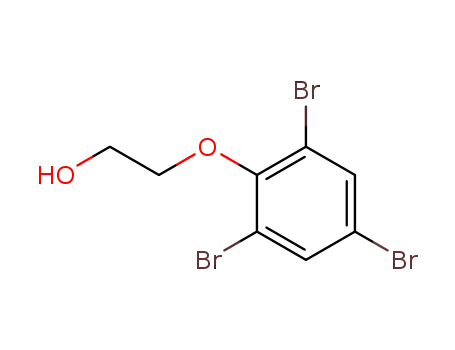
(2,4,6-tribromophenoxy)ethanol
-
607-99-8
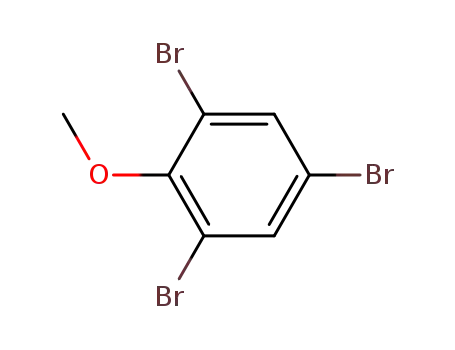
2,4,6-tribromoanisole
-
76461-14-8
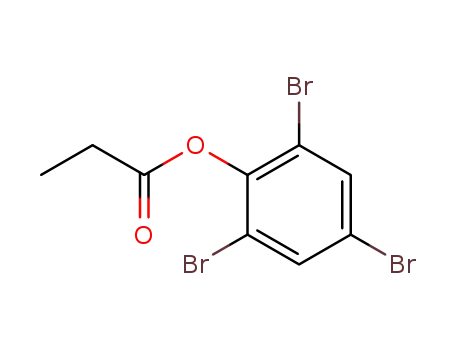
tribromophenyl propanoate
-
32019-71-9
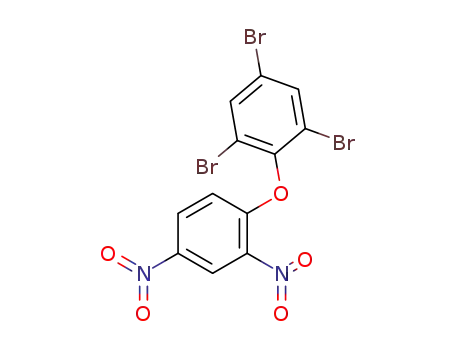
(2,4-dinitro-phenyl)-(2,4,6-tribromo-phenyl)-ether
Relevant Products
-
2-Bromo-2-nitro-1,3-propanediol
CAS:52-51-7
-
Mercaptopropyl Trimethoxysilane
CAS:3069-40-7