37942-01-1
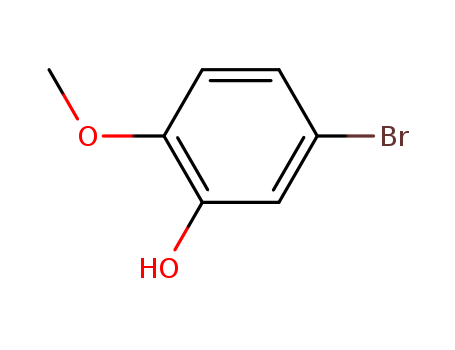
- Product Name:5-Bromo-2-Methoxyphenol
- Molecular Formula:C7H7BrO2
- Purity:99%
- Molecular Weight:203.035
Product Details;
CasNo: 37942-01-1
Molecular Formula: C7H7BrO2
Appearance: crystalline powder
Chinese Manufacturer supply 5-Bromo-2-Methoxyphenol 37942-01-1 in stock with high standard
- Molecular Formula:C7H7BrO2
- Molecular Weight:203.035
- Appearance/Colour:crystalline powder
- Vapor Pressure:0.00789mmHg at 25°C
- Melting Point:64-70 °C
- Refractive Index:1.578
- Boiling Point:259.7 °C at 760 mmHg
- PKA:9.01±0.10(Predicted)
- Flash Point:110.9 °C
- PSA:29.46000
- Density:1.585 g/cm3
- LogP:2.16330
5-Bromo-2-methoxyphenol(Cas 37942-01-1) Usage
InChI:InChI=1/C7H7BrO2/c1-10-7-3-2-5(8)4-6(7)9/h2-4,9H,1H3
37942-01-1 Relevant articles
Asymmetric Synthesis of β-Aryl β-Imido Sulfones Using Rhodium Catalysts with Chiral Diene Ligands: Synthesis of Apremilast
Syu, Jin-Fong,Gopula, Balraj,Jian, Jia-Hong,Li, Wei-Sian,Kuo, Ting-Shen,Wu, Ping-Yu,Henschke, Julian P.,Hsieh, Meng-Chi,Tsai, Ming-Kang,Wu, Hsyueh-Liang
, p. 4614 - 4618 (2019)
A chiral rhodium(I)-diene catalyst enabl...
Methods for preparation of apremilast
-
, (2021/05/19)
The present invention discloses a method...
Synthesis of a TNF inhibitor, flurbiprofen and an: I -Pr analogue in enantioenriched forms by copper-catalyzed propargylic substitution with Grignard reagents
Isogawa, Yukari,Kobayashi, Yuichi,Ogawa, Narihito,Takashima, Yuji,Tsuboi, Atsuki
, p. 9906 - 9909 (2021/12/07)
The copper-catalyzed substitution reacti...
Hollow, mesoporous, eutectic Zn1?xMgxO nano-spheres as solid acid-base catalysts for the highly regio-selectiveO-methylation of 1,2-diphenols
Liu, Jie,Ma, Xuebing,Wang, Xuri,Xie, Guangxin,Yin, Zuyong,Zhang, Jianing
, p. 7454 - 7466 (2021/11/23)
The highly regio-selectiveO-methylation ...
37942-01-1 Process route
-
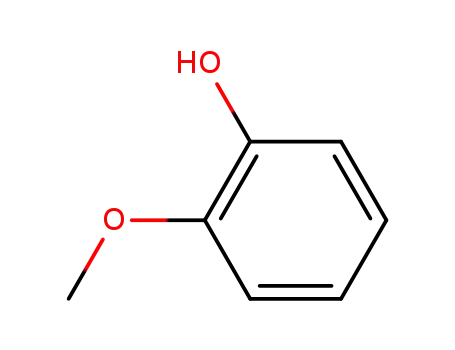
- 90-05-1
2-methoxy-phenol

-
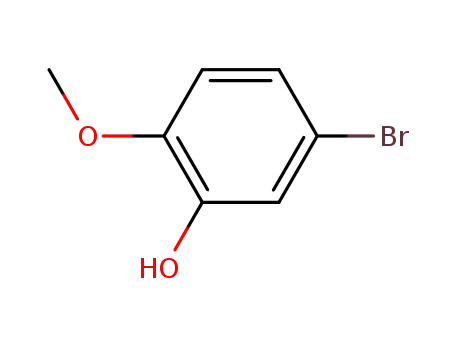
- 37942-01-1
5-bromo-2-methoxyphenol
| Conditions | Yield |
|---|---|
|
With sodium hydroxide; N-Bromosuccinimide; potassium tert-butylate; trifluoroacetic anhydride; In dichloromethane; acetonitrile;
|
90% |
|
2-methoxy-phenol; With methanesulfonyl chloride; zinc(II) chloride; at 120 ℃; for 1h; Inert atmosphere;
With hydrogen bromide; dihydrogen peroxide; In water; at 10 - 25 ℃; for 8h; Inert atmosphere;
With sodium hydroxide; In water; for 4h; Time; Reflux;
|
75.1% |
|
Multi-step reaction with 3 steps
1: 97 percent / H2SO4 / 6 h / 100 °C
2: 99 percent / NBS / acetonitrile / 10 h / 60 °C
3: 95 percent / NaHCO3 / methanol / 3 h / Heating
With N-Bromosuccinimide; sulfuric acid; sodium hydrogencarbonate; In methanol; acetonitrile;
|
|
|
Multi-step reaction with 3 steps
1: 98 percent / pyridine / ethyl acetate
2: Br2; AcOH / 20 °C
3: K2CO3 / methanol / 20 °C
With pyridine; bromine; potassium carbonate; acetic acid; In methanol; ethyl acetate;
|
|
|
Multi-step reaction with 2 steps
1: 51 percent / aq. NaOH / 3 h / 0 - 20 °C
2: 70 percent / Br2, AcOH / 48 h / Ambient temperature
With sodium hydroxide; bromine; acetic acid;
|
|
|
Multi-step reaction with 3 steps
1.1: zinc(II) chloride / 120 °C / Inert atmosphere
2.1: hydrogen bromide; dihydrogen peroxide / water / 8 h / 10 °C / Inert atmosphere
3.1: sodium hydroxide; water / 4 h / Reflux
3.2: pH 1
With water; hydrogen bromide; dihydrogen peroxide; sodium hydroxide; zinc(II) chloride; In water;
|
|
|
Multi-step reaction with 3 steps
1: sulfuric acid / 10 h / 25 - 100 °C
2: N-Bromosuccinimide / acetonitrile / 10 h / 60 °C / Inert atmosphere
3: sodium hydrogencarbonate / methanol; water / 3 h / Inert atmosphere; Reflux
With N-Bromosuccinimide; sulfuric acid; sodium hydrogencarbonate; In methanol; water; acetonitrile;
|
|
|
2-methoxy-phenol; With potassium tert-butylate; trifluoroacetic acid; In acetonitrile; at 20 ℃; for 0.75h;
With N-Bromosuccinimide; In acetonitrile; at 20 ℃;
|
|
|
Multi-step reaction with 2 steps
1: zinc(II) chloride / 1 h / 120 °C / Inert atmosphere
2: 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione / acetic acid / 3 h / 15 °C / Inert atmosphere
With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione; zinc(II) chloride; In acetic acid;
|
|
|
Multi-step reaction with 3 steps
1: zinc(II) chloride / 1 h / 120 °C / Inert atmosphere
2: hydrogen bromide; dihydrogen peroxide / water / 8 h / 10 - 25 °C / Inert atmosphere
3: sodium hydroxide / water / 4 h / Reflux
With hydrogen bromide; dihydrogen peroxide; sodium hydroxide; zinc(II) chloride; In water;
|
|
|
Multi-step reaction with 3 steps
1: triethylamine; dmap / dichloromethane / 0 - 20 °C
2: N-Bromosuccinimide / acetonitrile / 24 h / 60 °C / Inert atmosphere
3: potassium hydroxide / methanol / 5 h / Inert atmosphere; Reflux
With dmap; N-Bromosuccinimide; triethylamine; potassium hydroxide; In methanol; dichloromethane; acetonitrile;
|
|
|
2-methoxy-phenol; With trifluoroacetic anhydride; In acetonitrile; at 20 ℃; for 0.0833333h;
With potassium tert-butylate; In acetonitrile; at 20 ℃; for 0.75h;
With N-Bromosuccinimide; In acetonitrile; for 24h;
|
|
|
|
|
|
Multi-step reaction with 3 steps
1: sulfuric acid / water / 12 h / 20 - 100 °C / Inert atmosphere
2: N-Bromosuccinimide / acetonitrile / 11.5 h / 60 °C / Inert atmosphere
3: sodium hydrogencarbonate / methanol / 3 h / 70 °C / Inert atmosphere
With N-Bromosuccinimide; sulfuric acid; sodium hydrogencarbonate; In methanol; water; acetonitrile;
|
|
|
Multi-step reaction with 3 steps
1: sulfuric acid / water / 12 h / 100 °C
2: N-Bromosuccinimide / acetonitrile / 11.5 h / 60 °C
3: sodium hydrogencarbonate; water / methanol / 3 h / 70 °C
With N-Bromosuccinimide; sulfuric acid; water; sodium hydrogencarbonate; In methanol; water; acetonitrile;
|
|
|
Multi-step reaction with 3 steps
1: pyridine / 18 h / 20 °C
2: N-Bromosuccinimide / acetonitrile / 60 °C / Inert atmosphere
3: methanol; sodium hydrogencarbonate / 4 h / Reflux
With pyridine; methanol; N-Bromosuccinimide; sodium hydrogencarbonate; In acetonitrile;
|
-
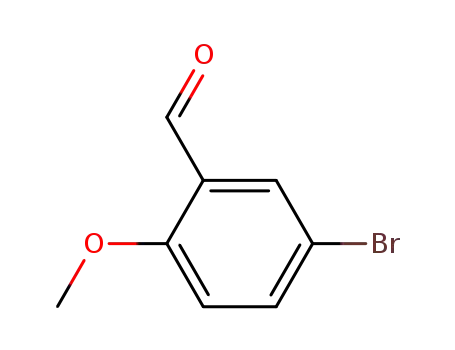
- 25016-01-7
5-bromo-2-methoxybenzaldehyde

-

- 37942-01-1
5-bromo-2-methoxyphenol
| Conditions | Yield |
|---|---|
|
With 3-chloro-benzenecarboperoxoic acid; In dichloromethane; at 20 ℃; for 48h;
|
92% |
|
5-bromo-2-methoxybenzaldehyde; With 3-chloro-benzenecarboperoxoic acid; In dichloromethane; at 0 - 20 ℃; for 72h;
With sodium hydroxide; In diethyl ether; Further stages.;
|
92% |
|
With sodium hydrogencarbonate; 3-chloro-benzenecarboperoxoic acid; In dichloromethane;
|
92% |
|
With 3-chloro-benzenecarboperoxoic acid; In dichloromethane; for 48h; Ambient temperature;
|
86% |
|
With 3-chloro-benzenecarboperoxoic acid; In dichloromethane; at 0 - 20 ℃; for 72h;
|
81.7% |
|
5-bromo-2-methoxybenzaldehyde; With 3-chloro-benzenecarboperoxoic acid; In dichloromethane; at 20 ℃; for 48h;
With sodium hydrogencarbonate; In dichloromethane; water;
With hydrogenchloride; lithium hydroxide; water; more than 3 stages;
|
72% |
|
With 3-chloro-benzenecarboperoxoic acid; In dichloromethane; at 20 ℃; for 48h;
|
72% |
|
5-bromo-2-methoxybenzaldehyde; With 3-chloro-benzenecarboperoxoic acid; In dichloromethane; at 20 ℃; for 48h;
With methanol; lithium hydroxide; water; for 0.166667h;
With hydrogenchloride; water; In methanol; pH=6 - 7;
|
72% |
|
5-bromo-2-methoxybenzaldehyde; With peracetic acid; acetic acid; In ethyl acetate; cooling;
With potassium hydroxide; In methanol; at 0 ℃; for 0.5h;
|
65% |
|
5-bromo-2-methoxybenzaldehyde; With peracetic acid; acetic acid; In ethyl acetate; at 20 ℃;
With potassium hydroxide; In methanol; at 0 ℃; for 0.5h;
|
65% |
|
With 3-chloro-benzenecarboperoxoic acid; In dichloromethane; at 0 - 20 ℃;
|
37% |
|
With peracetic acid; iron(II) sulfate; Yield given. Multistep reaction; 1.) acetic acid, room temp., 16 h; 2.) H2O, 20 min;
|
|
|
With sulfuric acid; dihydrogen peroxide; In methanol;
|
|
|
With 3-chloro-benzenecarboperoxoic acid;
|
|
|
Multi-step reaction with 2 steps
1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 5 h / 0 °C
2: sodium hydroxide / methanol; water / 0 °C
With 3-chloro-benzenecarboperoxoic acid; sodium hydroxide; In methanol; dichloromethane; water;
|
37942-01-1 Upstream products
-
21702-84-1
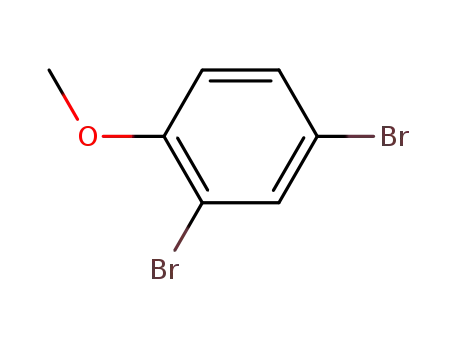
2,4-dibromoanisole
-
66037-04-5
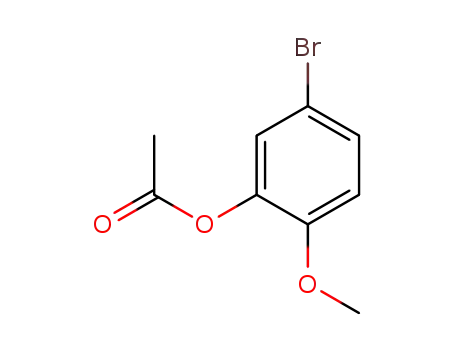
1-acetoxy-5-bromo-2-methoxybenzene
-
158429-08-4
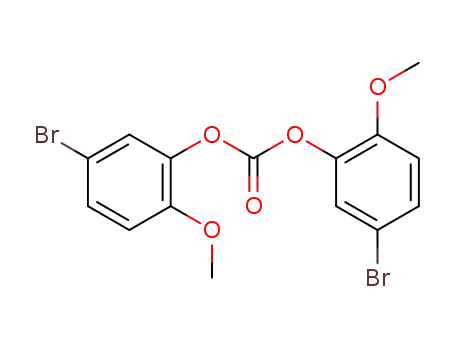
carbonic acid bis-(5-bromo-2-methoxy-phenyl ester)
-
1687-53-2
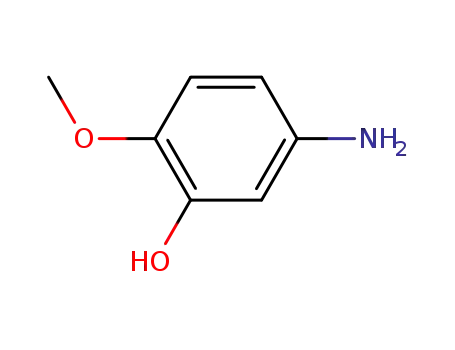
5-amino-2-methoxyphenol
37942-01-1 Downstream products
-
109514-41-2
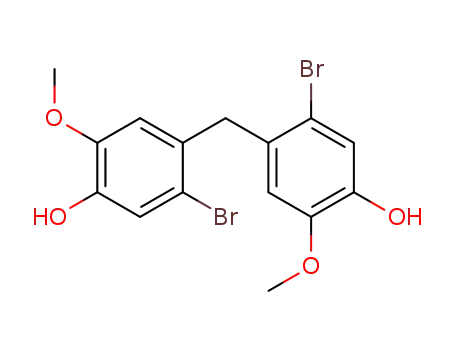
5,5'-dibromo-2,2'-dimethoxy-4,4'-methanediyl-di-phenol
-
38926-86-2
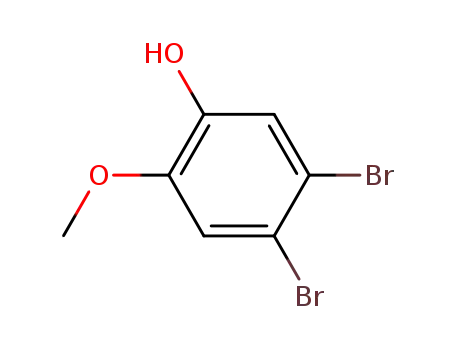
1,2-dibromo-4-hydroxy-5-methoxybenzene
-
645-08-9
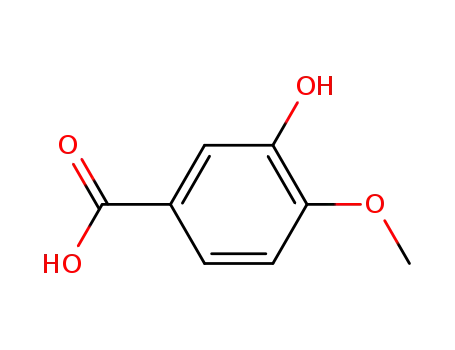
Isovanillic acid
-
861365-54-0
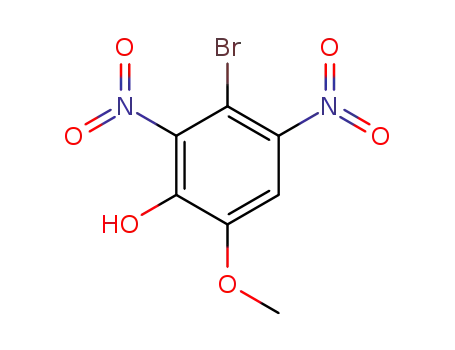
3-bromo-6-methoxy-2,4-dinitro-phenol
Relevant Products
-
2-Bromo-2-nitro-1,3-propanediol
CAS:52-51-7
-
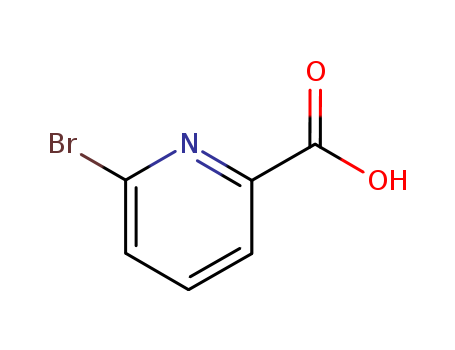
2-Bromo-6-Pyridinecarboxylic Acid
CAS:21190-87-4
-
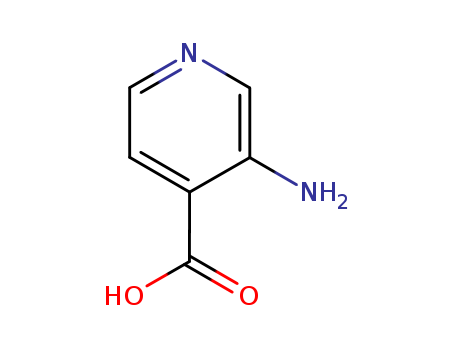
3-Amino-4-Pyridinecarboxylic Acid
CAS:7579-20-6