22901-09-3
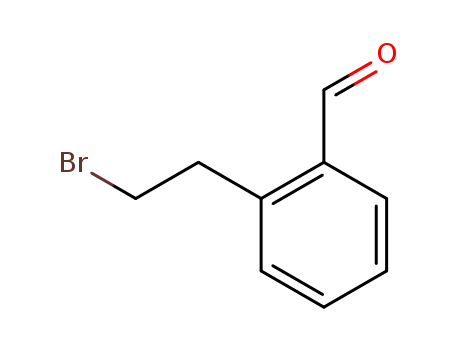
- Product Name:2-(2-Bromoethyl)benzaldehyde
- Molecular Formula:C9H9BrO
- Purity:99%
- Molecular Weight:213.074
Product Details;
CasNo: 22901-09-3
Molecular Formula: C9H9BrO
Factory Supply industrial standard 2-(2-Bromoethyl)benzaldehyde 22901-09-3 In Stock
- Molecular Formula:C9H9BrO
- Molecular Weight:213.074
- Vapor Pressure:0.006mmHg at 25°C
- Refractive Index:1.5858
- Boiling Point:273.74 °C at 760 mmHg
- Flash Point:81.557 °C
- PSA:17.07000
- Density:1.448 g/cm3
- LogP:2.43650
2-(2-Bromoethyl)benzaldehyde(Cas 22901-09-3) Usage
|
General Description |
2-(2-Bromoethyl)benzaldehyde is a chemical compound often used in organic synthesis. Its molecular formula is C9H9BrO. The primary feature of this compound is its aromatic structure combined with its bromine and aldehyde functional groups, which allows it to participate in a range of chemical reactions. Its properties include a molecular weight of approximately 227.07 g/mol and a heavy atom count of 11. Safety precautions must be followed while handling, as it can cause skin, eye and respiratory irritations. This chemical is often used in scientific research as a reagent. |
InChI:InChI=1/C9H9BrO/c10-6-5-8-3-1-2-4-9(8)7-11/h1-4,7H,5-6H2
22901-09-3 Relevant articles
Asymmetric synthesis of tetrahydroisoquinoline derivatives through 1, 3-dipolar cycloaddition of C, N-cyclic azomethine imines with allyl alkyl ketones
Chen, Wenyan,Feng, Guipeng,Ma, Guoyang,Wang, Kaikai,Wang, Shaoyan,Xu, Shaohong
, (2021)
[3 + 2] A 1, 3-Dipolar cycloaddition of ...
Dihydroisoquinolinium salts: Catalysts for asymmetric epoxidation
Page, Philip C. Bulman,Rassias, Gerasimos A.,Barros, David,Bethell, Donald,Schilling, Mark B.
, p. 3325 - 3334 (2000)
A range of dihydroisoquinolinium salts w...
Studies on Cu-catalyzed asymmetric alkynylation of tetrahydroisoquinoline derivatives
Li, Zhiping,MacLeod, Patricia D.,Li, Chao-Jun
, p. 590 - 597 (2006)
Enantioselective C-C bond formations bet...
Ytterbium-Catalyzed Intramolecular [3 + 2] Cycloaddition based on Furan Dearomatization to Construct Fused Triazoles
Xu, Xiaoming,Zhong, Ying,Xing, Qingzhao,Gao, Ziwei,Gou, Jing,Yu, Binxun
supporting information, p. 5176 - 5181 (2020/07/14)
The 1,2,3-triazole-containing polycyclic...
Hydrogen-Bonding-Promoted Cascade Rearrangement Involving the Enlargement of Two Rings: Efficient Access to Polycyclic Quinoline Derivatives
Cao, Wen-Bin,Ji, Shun-Jun,Lan, Yu,Li, Haiyan,Li, Shijun,Xu, Meng-Meng,Xu, Xiao-Ping
, p. 21425 - 21430 (2020/09/23)
An efficient cascade reaction of tryptam...
An Intramolecular Cycloaddition Approach to the Kauranoid Family of Diterpene Metabolites
Callebaut, Brenda,Hullaert, Jan,Van Hecke, Kristof,Winne, Johan M.
supporting information, p. 310 - 314 (2019/01/10)
Synthetic studies toward the ent-kaurano...
22901-09-3 Process route
-
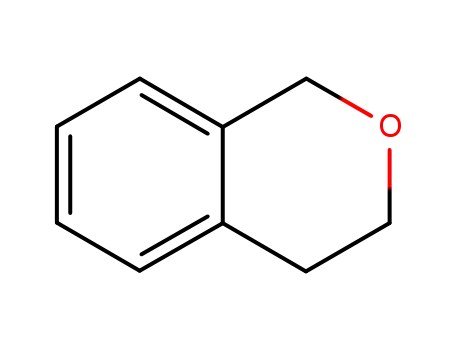
- 493-05-0
isochromane

-
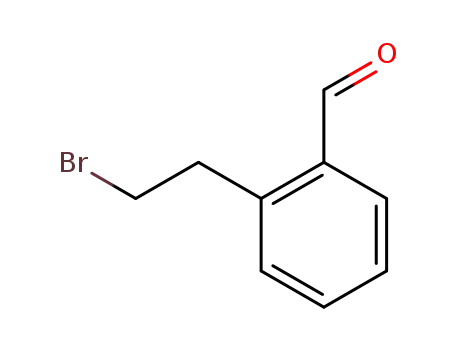
- 22901-09-3
2-(2-bromoethyl)benzaldehyde
| Conditions | Yield |
|---|---|
|
With bromine; In dichloromethane; at 38 - 40 ℃; for 4h; strong light source;
|
84.8% |
|
isochromane; With bromine; In tetrachloromethane; for 2h; Inert atmosphere; Reflux;
With hydrogen bromide; In water; for 0.333333h; Reflux; Inert atmosphere;
|
79% |
|
With copper(ll) bromide; In acetonitrile; for 1h; Inert atmosphere; Reflux;
|
75% |
|
With copper(ll) bromide; In acetonitrile; for 16h; Reflux; Inert atmosphere;
|
70% |
|
With copper(ll) bromide; In acetonitrile; Reagent/catalyst; Solvent; Temperature; Reflux; Inert atmosphere;
|
68% |
|
With copper(ll) bromide; In acetonitrile; Inert atmosphere; Reflux;
|
68% |
|
With copper(ll) bromide; In acetonitrile; Inert atmosphere; Reflux;
|
68% |
|
With copper(ll) bromide; In acetonitrile; for 28h; Reflux; Inert atmosphere;
|
43% |
|
With tetrachloromethane; bromine; unter UV-Belichtung und Erhitzen des Rktprod. mit wss. HBr;
|
|
|
isochromane; With bromine; Irradiation;
With silica gel; for 0.0166667h; Irradiation;
|
|
|
With bromine; In dichloromethane; Heating; sunlight irradiation;
|
|
|
isochromane; With bromine; In tetrachloromethane; for 1h; Heating;
With hydrogen bromide; In water; for 0.25h; Heating;
|
|
|
With bromine; sodium hydrogencarbonate; In dichloromethane;
|
|
|
Multi-step reaction with 2 steps
1: bromine / water / Irradiation
2: hydrogen bromide / Heating; Large scale
With hydrogen bromide; bromine; In water;
|
|
|
Multi-step reaction with 2 steps
1: 2,3-dicyano-5,6-dichloro-p-benzoquinone / dichloromethane / 20 °C
2: trimethylsilyl bromide; tetrabutylammomium bromide / toluene / 80 °C
With trimethylsilyl bromide; tetrabutylammomium bromide; 2,3-dicyano-5,6-dichloro-p-benzoquinone; In dichloromethane; toluene;
|
|
|
Multi-step reaction with 2 steps
1: 2,3-dicyano-5,6-dichloro-p-benzoquinone / dichloromethane / 48 h / 20 °C / Inert atmosphere
2: trimethylsilyl bromide; tetrabutylammomium bromide / toluene / 4 h / 80 °C / Inert atmosphere
With trimethylsilyl bromide; tetrabutylammomium bromide; 2,3-dicyano-5,6-dichloro-p-benzoquinone; In dichloromethane; toluene;
|
|
|
isochromane; With bromine; In tetrachloromethane; Reflux;
With hydrogen bromide; Reflux;
|
|
|
Multi-step reaction with 2 steps
1: 2,3-dicyano-5,6-dichloro-p-benzoquinone / dichloromethane / 48 h / 20 °C
2: trimethylsilyl bromide; tetrabutylammomium bromide / toluene / 4 h / 80 °C
With trimethylsilyl bromide; tetrabutylammomium bromide; 2,3-dicyano-5,6-dichloro-p-benzoquinone; In dichloromethane; toluene;
|
|
|
With copper(ll) bromide; In acetonitrile; Reflux;
|
|
|
isochromane; With bromine; In tetrachloromethane; for 1h; Reflux;
With hydrogen bromide; In water; for 0.25h; Reflux;
|
3.3 g |
|
Multi-step reaction with 2 steps
1: bromine / tetrachloromethane / Cooling with ice; Inert atmosphere; Reflux
2: hydrogen bromide / water / Inert atmosphere; Reflux
With hydrogen bromide; bromine; In tetrachloromethane; water;
|
|
|
Multi-step reaction with 2 steps
1: 2,3-dicyano-5,6-dichloro-p-benzoquinone / dichloromethane / 48 h / 20 °C
2: tetrabutylammomium bromide; trimethylsilyl bromide / toluene / 4 h / 80 °C
With trimethylsilyl bromide; tetrabutylammomium bromide; 2,3-dicyano-5,6-dichloro-p-benzoquinone; In dichloromethane; toluene;
|
|
|
Multi-step reaction with 2 steps
1: 2,3-dicyano-5,6-dichloro-p-benzoquinone / dichloromethane / 48 h / 25 °C
2: trimethylsilyl bromide; tetrabutylammomium bromide / toluene / 4 h / 80 °C
With trimethylsilyl bromide; tetrabutylammomium bromide; 2,3-dicyano-5,6-dichloro-p-benzoquinone; In dichloromethane; toluene;
|
|
|
With copper(ll) bromide; In acetonitrile; for 3h; Inert atmosphere; Reflux;
|
949 mg |
|
Multi-step reaction with 2 steps
1: 2,3-dicyano-5,6-dichloro-p-benzoquinone / dichloromethane / 24 h / 25 °C / Inert atmosphere
2: tetrabutylammomium bromide; trimethylsilyl bromide / toluene / 4 h / 80 °C
With trimethylsilyl bromide; tetrabutylammomium bromide; 2,3-dicyano-5,6-dichloro-p-benzoquinone; In dichloromethane; toluene;
|
-
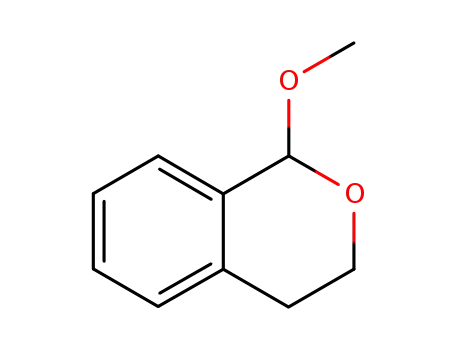
- 34818-49-0
1-methoxyisochromane

-

- 22901-09-3
2-(2-bromoethyl)benzaldehyde
| Conditions | Yield |
|---|---|
|
With trimethylsilyl bromide; tetrabutylammomium bromide; In toluene; at 80 ℃; for 4h;
|
71% |
|
With trimethylsilyl bromide; tetrabutylammomium bromide; In toluene; at 80 ℃;
|
|
|
With trimethylsilyl bromide; tetrabutylammomium bromide; In toluene; at 80 ℃; for 4h; Inert atmosphere;
|
|
|
With trimethylsilyl bromide; tetrabutylammomium bromide; In toluene; at 80 ℃; for 4h;
|
|
|
With trimethylsilyl bromide; tetrabutylammomium bromide; In toluene; at 80 ℃; for 4h;
|
|
|
With trimethylsilyl bromide; tetrabutylammomium bromide; In toluene; at 80 ℃; for 4h;
|
|
|
With trimethylsilyl bromide; tetrabutylammomium bromide; In toluene; at 80 ℃; for 4h;
|
22901-09-3 Upstream products
-
493-05-0

isochromane
-
50683-32-4
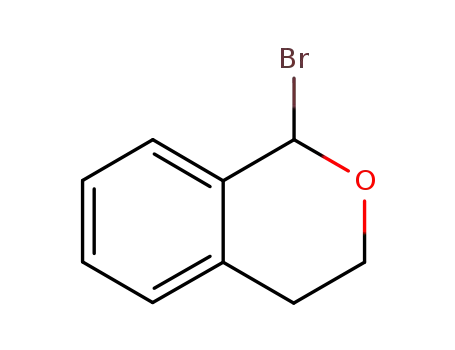
1-Bromoisochromane
-
19275-61-7
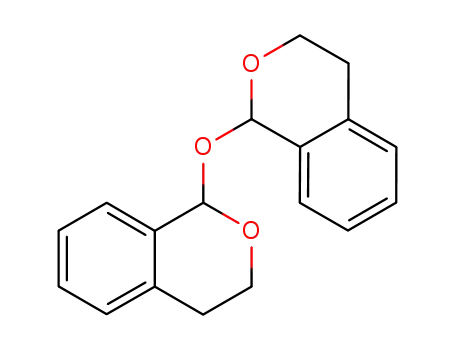
bis(3,4-dihydro-1H-benzopyran-1-yl) ether
-
10035-10-6
hydrogen bromide
22901-09-3 Downstream products
-
75802-22-1
1-ethoxyisochromane
-
24423-87-8
3,4-dihydro-isoquinoline 2-oxide
-
95071-86-6
2,3,5,6-tetrahydro-10bH-oxazolo<2,3-a>isoquinoline
-
5811-08-5
2,3,4,6,7,11b-hexahydro-[1,3]oxazino[2,3-a]isoquinoline
Relevant Products
-
2-Bromo-2-nitro-1,3-propanediol
CAS:52-51-7
-
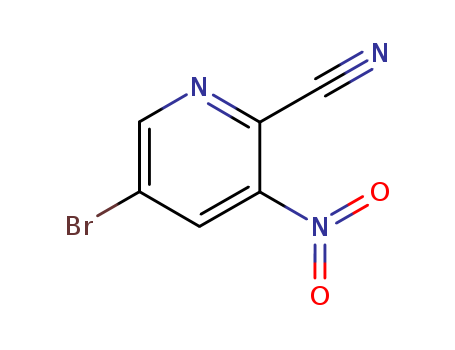
5-Bromo-2-Cyano-3-Nitropyridine
CAS:573675-25-9
-
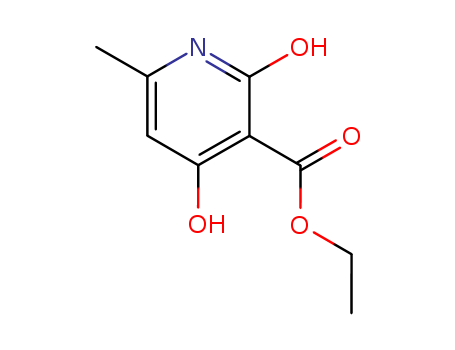
2,4-Dihydroxy-6-Methyl-Nicotinic Acid Ethyl Ester
CAS:70254-52-3