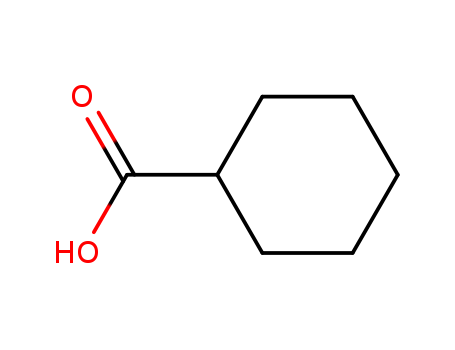
98-89-5
- Product Name:Cyclohexane Carboxylic Acid
- Molecular Formula:C7H12O2
- Purity:99%
- Molecular Weight:128.171
Product Details;
CasNo: 98-89-5
Molecular Formula: C7H12O2
Appearance: white crystalline solid
Purity 99% Min Cyclohexane Carboxylic Acid 98-89-5 Spot Supply with Safe Transportation
- Molecular Formula:C7H12O2
- Molecular Weight:128.171
- Appearance/Colour:white crystalline solid
- Vapor Pressure:0.02mmHg at 25°C
- Melting Point:29-31 °C
- Refractive Index:1.460 - 1.462
- Boiling Point:233 °C at 760 mmHg
- PKA:4.9(at 25℃)
- Flash Point:107 °C
- PSA:37.30000
- Density:1.079 g/cm3
- LogP:1.65130
Cyclohexanecarboxylic acid(Cas 98-89-5) Usage
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 22, p. 1680, 1957 DOI: 10.1021/jo01363a041Tetrahedron Letters, 21, p. 4773, 1980 DOI: 10.1016/0040-4039(80)80136-5 |
|
Biochem/physiol Actions |
Cyclohexanecarboxylic acid undergoes microbial degradation by a strain of Antherobacter to form para-hydroxybenzoic acid. Cyclohexanecarboxylic acid undergoes aromatization and converts to Hippuric acid in rat liver extracts in vitro. Cyclohexanecarboxylic acid is the starting reagent for the synthesis of polyketide-type antibiotics, Phoslactomycins. |
|
Purification Methods |
Crystallise the acid from hot H2O (solubility is 0.2% w/w at 15o), it is soluble in organic solvents. Also distil it at as high a vacuum as possible and warm the condenser as it solidifies on cooling.The acid chloride M 146.6, has b 184o/atm, d2 5 1.096, the methyl ester has b 183o/atm, and the S-benzylisothiuronium salt has m 165-166o (from EtOH). [Beilstein 9 H 7, 9 I 5, 9 II 6, 9 III15, 9 IV 16.] |
|
Application |
Cyclohexanecarboxylic acid is a precursor to the nylon-6 precursor caprolactam via its reaction with nitrosylsulfuric acid. It can also be oxidized to cyclohexene. Cyclohexanecarboxylic acid exhibits the reactions typical of carboxylic acids, including its conversion to the acid chloride cyclohexanecarbonyl chloride. |
|
Definition |
ChEBI: A monocarboxylic acid that consists of cyclohexane substituted by a carboxy group. |
|
Taste threshold values |
Taste characteristics at 5 ppm: fruity, woody, berry-like with green dirty nuances |
|
General Description |
Cyclohexanecarboxylic acid, the saturated analog of benzoic acid, is categorized under the family of volatile organic compounds (VOCs). It is typically used as a flavoring ingredient. |
InChI:InChI=1/C7H12O2/c8-7(9)6-4-2-1-3-5-6/h6H,1-5H2,(H,8,9)
98-89-5 Relevant articles
Palladium and copper-catalyzed carboxylation of alkanes with carbon monoxide. Remarkable effect of the mixed metal salts
Nakata,Miyata,Jintoku,Kitani,Taniguchi,Takaki,Fujiwara
, p. 3755 - 3759 (1993)
The mixed catalyst Pd(OAc)2-Cu(OAc)2 pro...
Ring hydrogenation of aromatic compounds in aqueous suspensions of an Rh-loaded TiO2 photocatalyst without use of H2 gas
Nakanishi, Kousuke,Yagi, Ryosuke,Imamura, Kazuya,Tanaka, Atsuhiro,Hashimoto, Keiji,Kominami, Hiroshi
, p. 139 - 146 (2018)
There are various possibilities of co-ca...
Palladium Catalyzed Carboxylation of Cyclohexane with Carbon Monoxide
Nakata, Kazuyuki,Watanabe, Jun,Takaki, Ken,Fujiwara, Yuzo
, p. 1437 - 1438 (1991)
Very high turnover numbers of the cataly...
Boosting Catalysis of Pd Nanoparticles in MOFs by Pore Wall Engineering: The Roles of Electron Transfer and Adsorption Energy
Chen, Dongxiao,Yang, Weijie,Jiao, Long,Li, Luyan,Yu, Shu-Hong,Jiang, Hai-Long
, (2020)
The chemical environment of metal nanopa...
In-situ generated highly dispersed nickel nanoclusters confined in MgAl mixed metal oxide platelets for benzoic acid hydrogenation
Zhang, Huiling,Dong, Jie,Qiao, Xianliang,Qin, Jingru,Sun, Haofei,Wang, Aiqing,Niu, Libo,Bai, Guoyi
, p. 258 - 265 (2019)
A new and cost-effective NiMgAl mixed me...
Highly effective Ir-based catalysts for benzoic acid hydrogenation: Experiment- and theory-guided catalyst rational design
Tang, Minghui,Mao, Shanjun,Li, Xuefeng,Chen, Chunhong,Li, Mingming,Wang, Yong
, p. 1766 - 1774 (2017)
On the way to exploring superior hydroge...
RuPd alloy nanoparticles supported on N-doped carbon as an efficient and stable catalyst for benzoic acid hydrogenation
Tang, Minghui,Mao, Shanjun,Li, Mingming,Wei, Zhongzhe,Xu, Fan,Li, Haoran,Wang, Yong
, p. 3100 - 3107 (2015)
RuPd alloy nanoparticles (3.6 nm) unifor...
A highly dispersed and stable Ni/mSiO2-AE nanocatalyst for benzoic acid hydrogenation
Zhang, Huiling,Gao, Xuejia,Ma, Yuanyuan,Han, Xue,Niu, Libo,Bai, Guoyi
, p. 5993 - 5999 (2017)
A Ni/mSiO2-AE nanocatalyst was successfu...
Multifunctional solid surfaces for enhanced catalysis
Motokura, Ken
, p. 3067 - 3068 (2014)
-
Gram-scale synthesis of carboxylic acids via catalytic acceptorless dehydrogenative coupling of alcohols and hydroxides at an ultralow Ru loading
Chen, Cheng,Cheng, Hua,Verpoort, Francis,Wang, Zhi-Qin,Wu, Zhe,Yuan, Ye,Zheng, Zhong-Hui
, (2021/12/13)
Acceptorless dehydrogenative coupling (A...
Transformation of Thioacids into Carboxylic Acids via a Visible-Light-Promoted Atomic Substitution Process
Fu, Qiang,Liang, Fu-Shun,Lou, Da-Wei,Pan, Gao-Feng,Wang, Rui,Wu, Min,Xie, Kai-Jun
, p. 2020 - 2024 (2022/03/31)
A visible-light-promoted atomic substitu...
Synthesis of β-nitro ketones from geminal bromonitroalkanes and silyl enol ethers by visible light photoredox catalysis
Cao, Haoying,Ma, Shanshan,Feng, Yanhong,Guo, Yawen,Jiao, Peng
supporting information, p. 1780 - 1783 (2022/02/17)
Various β-nitro ketones, including those...
Mechanochemical Grignard Reactions with Gaseous CO2 and Sodium Methyl Carbonate**
Pfennig, Victoria S.,Villella, Romina C.,Nikodemus, Julia,Bolm, Carsten
supporting information, (2022/01/22)
A one-pot, three-step protocol for the p...
98-89-5 Process route
-
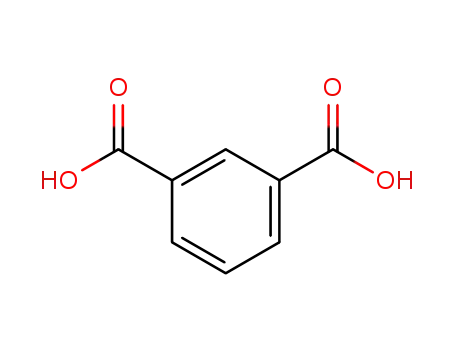
- 121-91-5
isophthalic acid

-
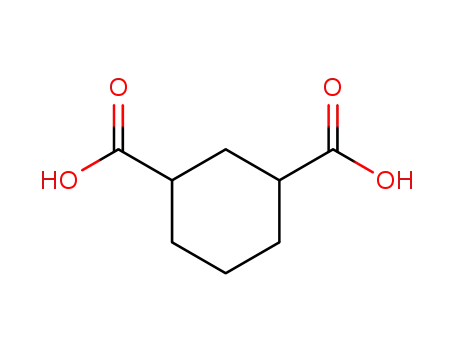
- 3971-31-1
cyclohexane-1,3-dicarboxylic acid

-
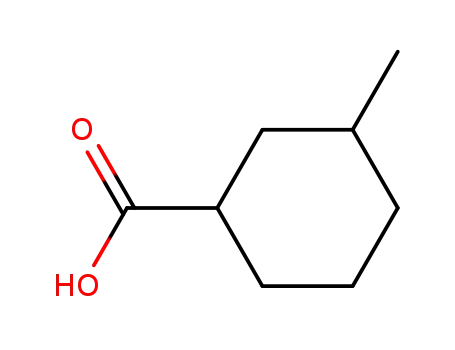
- 13293-59-9
3-methylcyclohexanecarboxylic acid

-
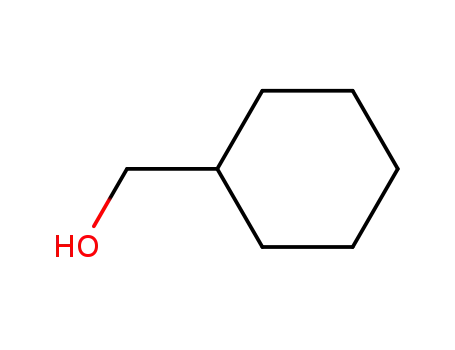
- 100-49-2
cyclohexylmethyl alcohol

-
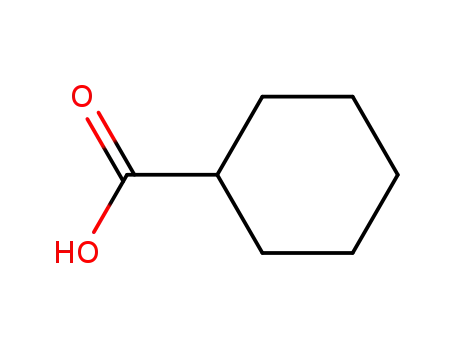
- 98-89-5
Cyclohexanecarboxylic acid
| Conditions | Yield |
|---|---|
|
With 5% active carbon-supported ruthenium; hydrogen; In 1,4-dioxane; at 219.84 ℃; for 12h; under 51680.2 Torr;
|
-
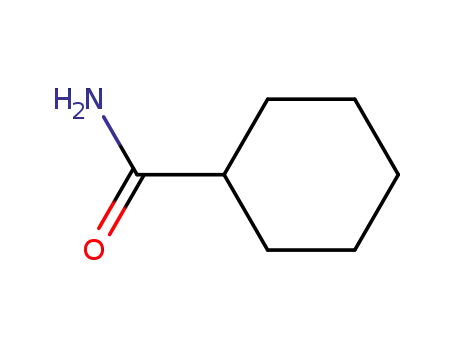
- 1122-56-1
cyclohexylcarboxamide

-

- 100-49-2
cyclohexylmethyl alcohol

-

- 98-89-5
Cyclohexanecarboxylic acid
| Conditions | Yield |
|---|---|
|
With water; hydrogen; at 59.84 ℃; for 4h; under 60006 Torr; Reagent/catalyst; Temperature; Pressure; Autoclave; Sealed tube;
|
98-89-5 Upstream products
-
766-66-5
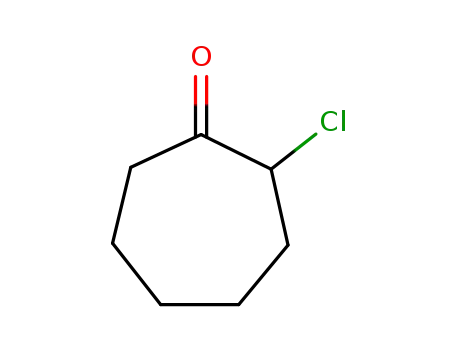
2-chlorocycloheptanone
-
1123-28-0
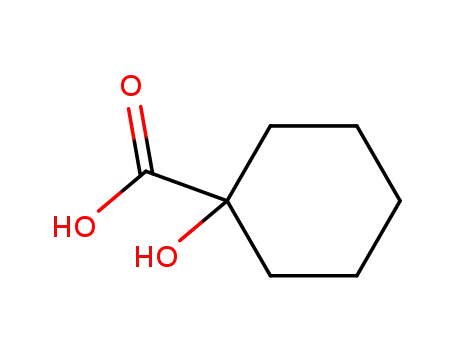
1-hydroxy-cyclohexanecarboxylic acid
-
2345-34-8
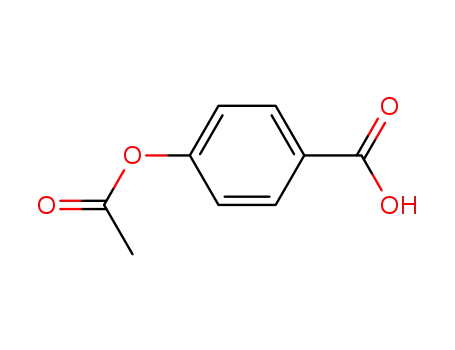
4-acetyloxy-benzoic acid
-
626-62-0
Cyclohexyl iodide
98-89-5 Downstream products
-
36978-20-8
cyclohexanecarboxylic acid-(4-chloro-butyl ester)
-
67238-99-7
1-cyclohexanecarbonyloxy-3-(2-methyl-piperidino)-propane; hydrochloride
-
22651-87-2
cyclohexanecarboxylic acid anhydride
-
766-05-2

cyclohexane carbonitrile
Relevant Products
-
5-(2′-Hydroxy-3′-Naphthamide)-2-Benzimidazolone
CAS:26848-40-8
-
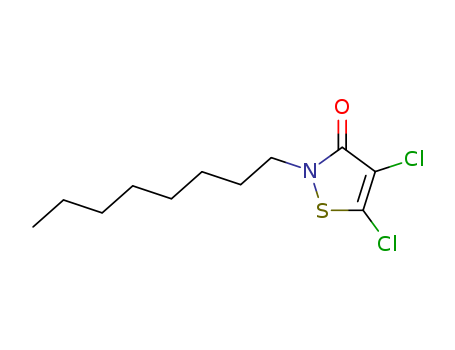
4,5-Dichloro-2-N-Octyl-4-Isothiazolin-3-One
CAS:64359-81-5
-
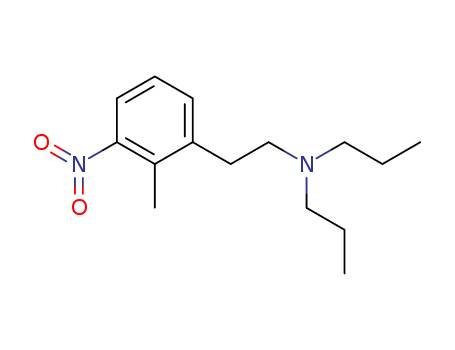
N,N-Diethyl-2-Methyl-3-Nitrophenylethylamine
CAS:91374-23-1