621-59-0
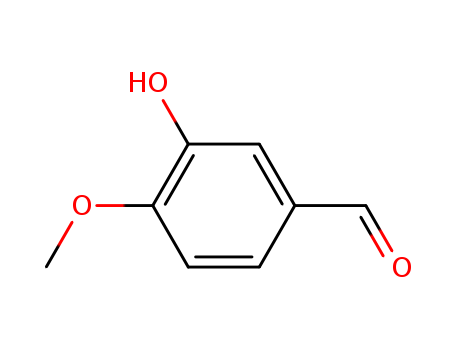
- Product Name:IsoVanillin
- Molecular Formula:C8H8O3
- Purity:99%
- Molecular Weight:152.15
Product Details;
CasNo: 621-59-0
Molecular Formula: C8H8O3
Appearance: light tan cyrstalline solid
Chinese Manufacturer Supply IsoVanillin 621-59-0 On Stock with Competitive Price
- Molecular Formula:C8H8O3
- Molecular Weight:152.15
- Appearance/Colour:light tan cyrstalline solid
- Vapor Pressure:0.000384mmHg at 25°C
- Melting Point:113-116 °C
- Refractive Index:1.587
- Boiling Point:308.1 °C at 760 mmHg
- PKA:pK1:8.889 (25°C)
- Flash Point:119.9 °C
- PSA:46.53000
- Density:1.231 g/cm3
- LogP:1.21330
Isovanillin(Cas 621-59-0) Usage
|
Preparation |
Synthesis of isovanillin: 4-Hydroxybenzaldehyde is used as raw material, firstly react with bromine to obtain 3-bromo-4-hydroxybenzaldehyde, then react with methyl iodide to obtain 3-bromo-4-methoxybenzaldehyde, and then hydrolyzed under the action of sodium hydroxide and cuprous chloride to produce isovanillin. The yield was 64.1%. |
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 45, p. 1596, 1980 DOI: 10.1021/jo01297a010 |
|
Flammability and Explosibility |
Notclassified |
|
Purification Methods |
Crystallise isovaniline from H2O or *C6H6. The oxime has m 147o. [Beilstein 8 IV 1764.] |
|
Application |
Isovanillin is a kind of fragrance and isomer of vanillin. it has unique properties than vanillin, its fragrance can change with the change of ambient temperature, so it is especially suitable for special cosmetics and some special fragrance industries. Isovanillin is a selective inhibitor of aldehyde oxidase. It is not a substrate of that enzyme, and is metabolized by aldehyde dehydrogenase into isovanillic acid, which could make it a candidate drug for use in alcohol aversion therapy. |
|
General Description |
3-Hydroxy-4-methoxybenzaldehyde on condensation with furan-2-carboxylic acid hydrazide and thiophene-2-carboxylic acid hydrazide yields Schiff-bases. It undergoes condensation reaction with1-azabicyclo[2.2.2]octan-3-one to give (Z)-2-(3-hydroxy-4-methoxybenzylidene)-1-azabicyclo[2.2.2]octan-3-one. |
InChI:InChI=1/C8H8O3/c1-11-8-3-2-6(5-9)4-7(8)10/h2-5,10H,1H3
621-59-0 Relevant articles
One-Pot Biocatalytic In Vivo Methylation-Hydroamination of Bioderived Lignin Monomers to Generate a Key Precursor to L-DOPA
Birmingham, William R.,Galman, James L.,Parmeggiani, Fabio,Seibt, Lisa,Turner, Nicholas J.
, (2022/01/13)
Electron-rich phenolic substrates can be...
Preparation method of 3-hydroxy-4-methoxyphenylpropylaldehyde
-
Paragraph 0048-0054; 0058-0065; 0068-0075; 0078-0085, (2021/11/10)
The invention provides a preparation met...
Method for synthesizing isovanillin
-
Paragraph 0019; 0031-0039; 0041-0051, (2021/07/31)
The invention provides a method for synt...
Thiols Act as Methyl Traps in the Biocatalytic Demethylation of Guaiacol Derivatives
Grimm, Christopher,Kroutil, Wolfgang,Pompei, Simona,Schiller, Christine,Schober, Lukas
supporting information, p. 16906 - 16910 (2021/07/02)
Demethylating methyl phenyl ethers is ch...
621-59-0 Process route
-
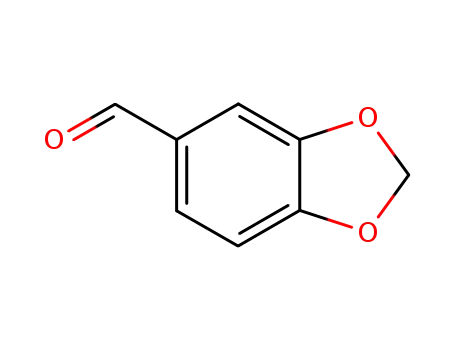
- 120-57-0,30024-74-9
piperonal

-
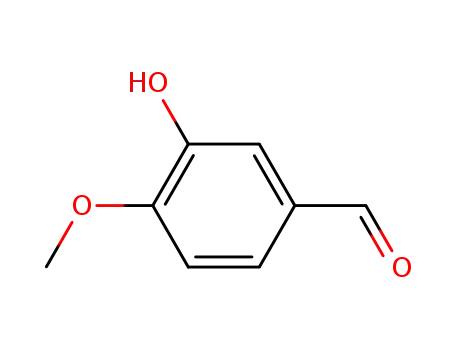
- 621-59-0
isovanillin

-
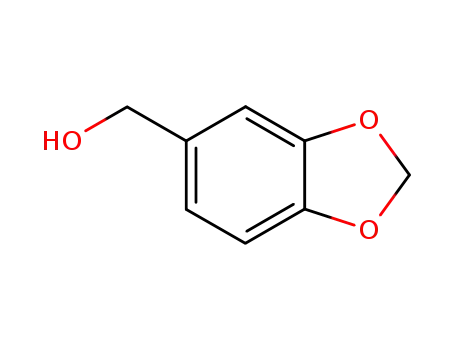
- 495-76-1
piperonol

-
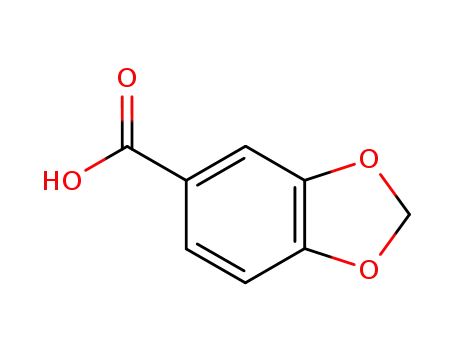
- 94-53-1
Piperonylic acid

-
![4-(Benzo[1,3]dioxol-5-ylmethoxy)-3-hydroxy-benzaldehyde](/upload/2025/1/0916530d-fa24-4f80-95b0-cc4e3fdb0e3a.png)
- 75437-95-5
4-(Benzo[1,3]dioxol-5-ylmethoxy)-3-hydroxy-benzaldehyde
| Conditions | Yield |
|---|---|
|
With sodium methylate; In dimethyl sulfoxide; at 150 ℃;
|
18.8% 17.6% 14.5% 18% |
|
With sodium methylate; In dimethyl sulfoxide; at 150 ℃;
|
18.8% 17.6% 18% 14.5% |
-

- 120-57-0,30024-74-9
piperonal

-
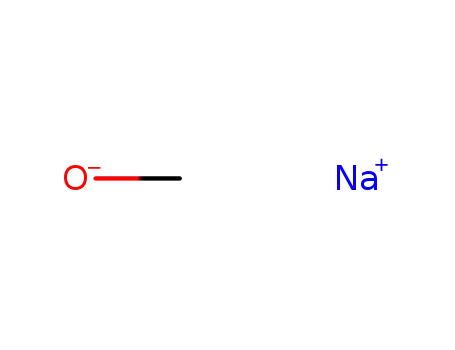
- 124-41-4
sodium methylate

-

- 621-59-0
isovanillin

-

- 495-76-1
piperonol

-

- 94-53-1
Piperonylic acid

-
![4-(Benzo[1,3]dioxol-5-ylmethoxy)-3-hydroxy-benzaldehyde](/upload/2025/1/0916530d-fa24-4f80-95b0-cc4e3fdb0e3a.png)
- 75437-95-5
4-(Benzo[1,3]dioxol-5-ylmethoxy)-3-hydroxy-benzaldehyde
| Conditions | Yield |
|---|---|
|
In dimethyl sulfoxide; at 150 ℃;
|
17.6% 18% 14.5% 18.8% |
621-59-0 Upstream products
-
1131-52-8
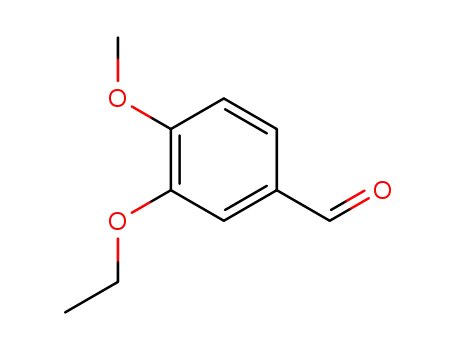
3-ethoxy-4-methoxybenzaldehyde
-
19784-98-6
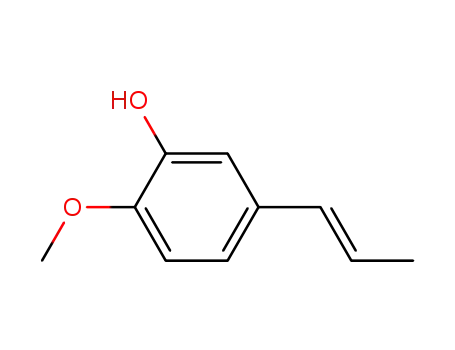
Isochavibetol
-
3207-31-6
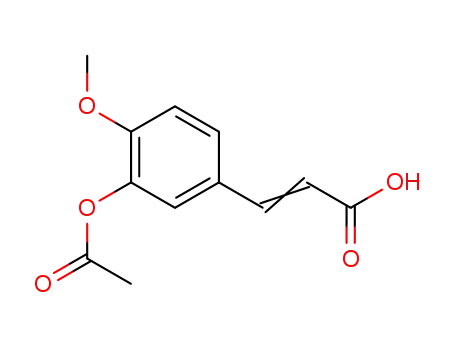
3-(3-acetoxy-4-methoxyphenyl)acrylic acid
-
519-05-1
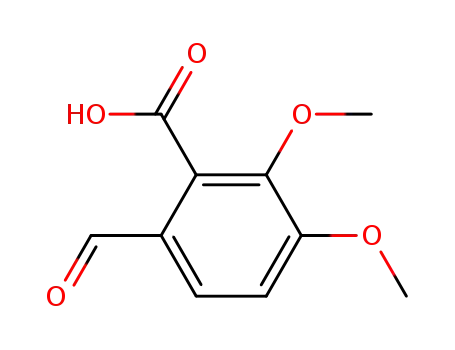
opianic acid
621-59-0 Downstream products
-
60655-15-4
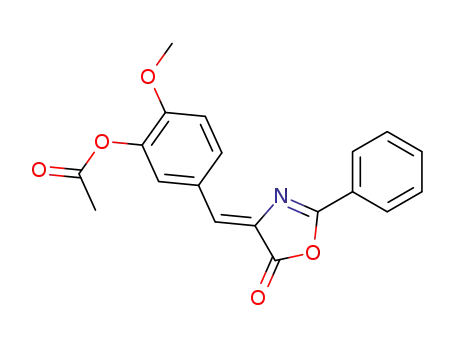
(Z)-2-methoxy-5-((5-oxo-2-phenyloxazol-4(5H)-ylidene)methyl)phenyl acetate
-
19784-98-6
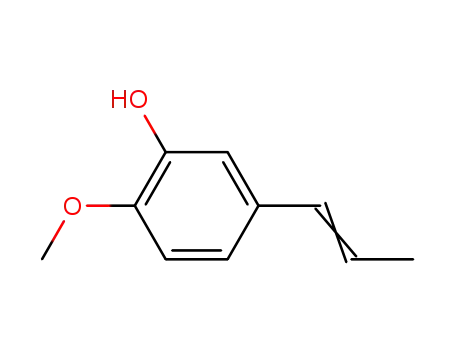
Isochavibetol
-
1131-52-8

3-ethoxy-4-methoxybenzaldehyde
-
104236-79-5
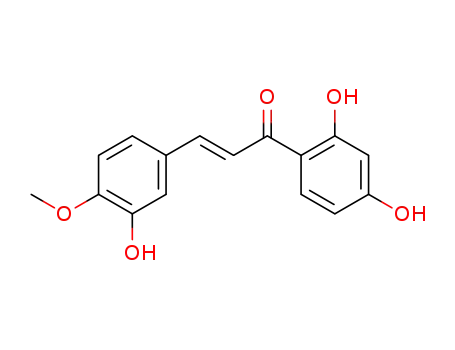
(E)-1-(2,4-dihydroxyphenyl)-3-(3-hydroxy-4-methoxyphenyl)-prop-2-en-1-one
Relevant Products
-
5-(2′-Hydroxy-3′-Naphthamide)-2-Benzimidazolone
CAS:26848-40-8
-
Fatty Alcohol Ethoxylated 7MOL
CAS:68439-50-9
-
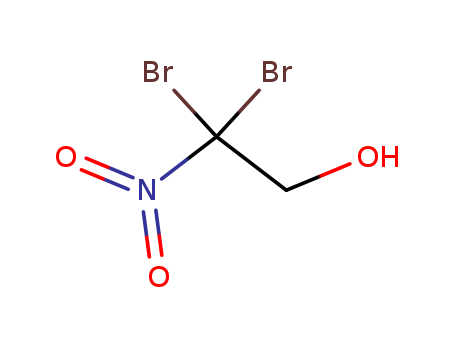
2,2-Dibromo-2-Nitroethanol
CAS:69094-18-4