111128-12-2
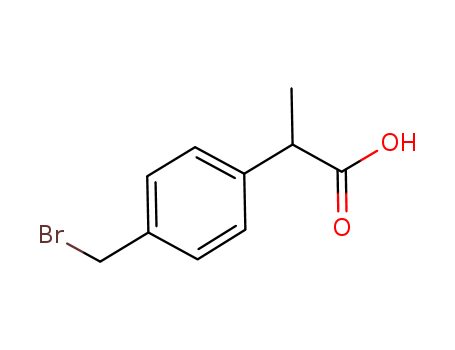
- Product Name:4-Bromomethylphenylacetic Acid
- Molecular Formula:C10H11BrO2
- Purity:99%
- Molecular Weight:243.1
Product Details;
CasNo: 111128-12-2
Molecular Formula: C10H11BrO2
Appearance: Beige-cream crystalline powder
Buy reliable Quality 4-Bromomethylphenylacetic Acid 111128-12-2 raw material with Honest Price
- Molecular Formula:C10H11BrO2
- Molecular Weight:243.1
- Appearance/Colour:Beige-cream crystalline powder
- Vapor Pressure:2.54E-05mmHg at 25°C
- Melting Point:126-130 °C(lit.)
- Refractive Index:1.5220 (estimate)
- Boiling Point:344.2 °C at 760 mmHg
- PKA:4.29±0.10(Predicted)
- Flash Point:162 °C
- PSA:37.30000
- Density:1.485 g/cm3
- LogP:2.76960
2-(4-Bromomethyl)phenylpropionic acid(Cas 111128-12-2) Usage
|
General Description |
2-[4-(Bromomethyl)phenyl]propionic acid is a propionic acid derivative. Its enthalpy of vaporization at boiling point (421.15K) is 36.363kjoule/mol and density at 25°C is 1.4212g/ml. |
InChI:InChI=1/C10H11BrO2/c1-7(10(12)13)9-4-2-8(6-11)3-5-9/h2-5,7H,6H2,1H3,(H,12,13)/p-1/t7-/m1/s1
111128-12-2 Relevant articles
Design, synthesis, and evaluation of novel 2-phenylpropionic acid derivatives as dual COX inhibitory-antibacterial agents
Karaca Gen?er, Hülya,Acar ?evik, Ulviye,Kaya ?avu?o?lu, Betül,Sa?l?k, Begüm Nurpelin,Levent, Serkan,Atl?, ?zlem,Ilg?n, Sinem,?zkay, Yusuf,Kaplanc?kl?, Zafer As?m
, p. 732 - 745 (2017)
-
Method for synthesizing P-bromomethyl isobenzopropionic acid
-
, (2021/10/27)
The invention provides a method for synt...
Preparation method of p-bromomethyl isophenylpropionic acid
-
Paragraph 0014; 0015; 0044; 0049; 0050; 0051; 0056; 0057, (2020/08/09)
The invention belongs to the field of sy...
Production method of 2-(4-bromomethyl phenyl)propionic acid
-
Paragraph 0017; 0022; 0028; 0029, (2020/03/06)
The invention discloses a production met...
Liquid-phase circulation preparation method for 2-(4-bromomethylphenyl)propionic acid
-
Paragraph 0063-0075; 0077; 0089-0095; 0097-0099; 0101-0103, (2019/01/22)
The invention discloses a liquid-phase c...
111128-12-2 Process route
-
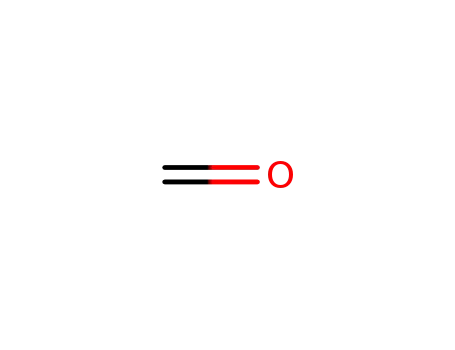
- 50-00-0,30525-89-4,61233-19-0
formaldehyd

-
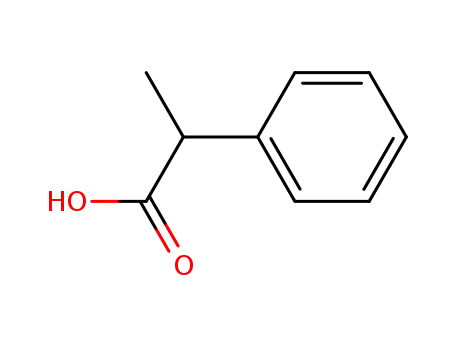
- 492-37-5,2328-24-7
hydratropic acid

-
![2-[4-(bromomethyl)phenyl]propanoic acid](/upload/2025/1/77a5ff82-df7a-4201-9aad-8c808a3c50c1.png)
- 111128-12-2
2-[4-(bromomethyl)phenyl]propanoic acid
| Conditions | Yield |
|---|---|
|
With sulfuric acid; hydrogen bromide; at 35 - 70 ℃; for 11h; Temperature;
|
99% |
|
With sulfuric acid; hydrogen bromide; at 35 - 90 ℃; for 11.5h; Temperature;
|
96% |
|
formaldehyd; hydratropic acid; With hydrogen bromide; at 55 - 65 ℃;
With sulfuric acid; at 20 - 65 ℃;
|
96% |
|
With 1-dodecyl-3-methylimidazol-1-ium bromide; hydrogen bromide; In water; at 60 ℃; for 6h; Reagent/catalyst;
|
87% |
|
With hydrogen bromide; phosphorus tribromide; zinc dibromide; In n-heptane; water; at 80 - 90 ℃; for 14h;
|
86% |
|
With hydrogen bromide; phosphorus tribromide; zinc dibromide; In water; at 80 - 90 ℃; for 10h; Concentration; Reagent/catalyst; Temperature; Time;
|
86.7% |
|
With sulfuric acid; hydrogen bromide; at 50 - 120 ℃; for 3h; Time; Temperature;
|
80.8% |
-
-
C10H10Br2O2

-
![2-[4-(bromomethyl)phenyl]propanoic acid](/upload/2025/1/77a5ff82-df7a-4201-9aad-8c808a3c50c1.png)
- 111128-12-2
2-[4-(bromomethyl)phenyl]propanoic acid
| Conditions | Yield |
|---|---|
|
With tetra-(n-butyl)ammonium iodide; phosphonic acid diethyl ester; In water; toluene; at 5 - 85 ℃; for 6h; Reagent/catalyst; Inert atmosphere;
|
85.3% |
111128-12-2 Upstream products
-
188680-60-6
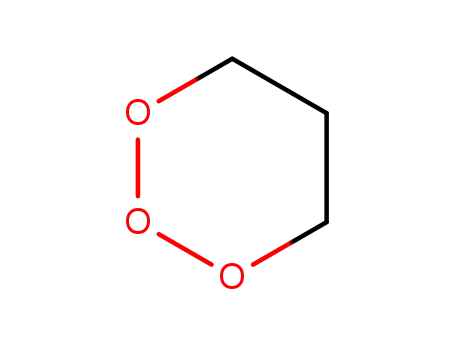
trioxane
-
492-37-5

hydratropic acid
-
57-09-0

cetyltrimethylammonim bromide
-
50-00-0
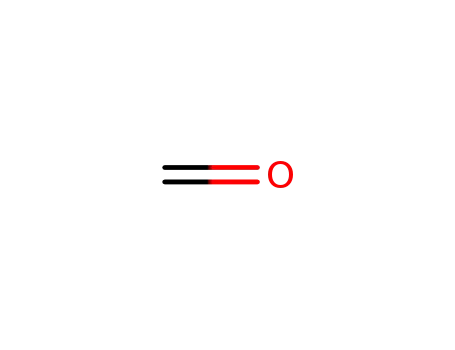
formaldehyd
111128-12-2 Downstream products
-
35981-81-8
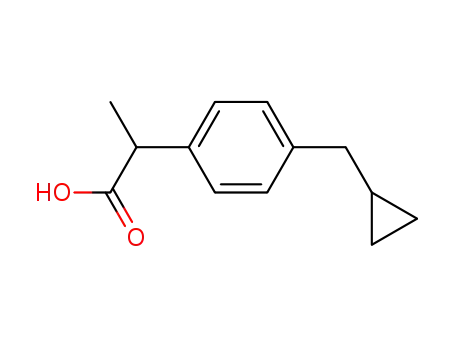
p-(Cyclopropylmethyl)-hydratropinsaeure
-
68266-50-2
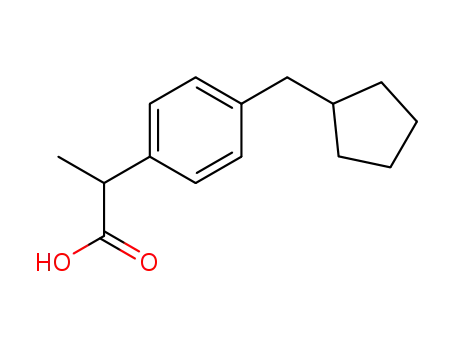
2-<4-(cyclopentylmethyl)phenyl>propionic acid
-
99807-54-2
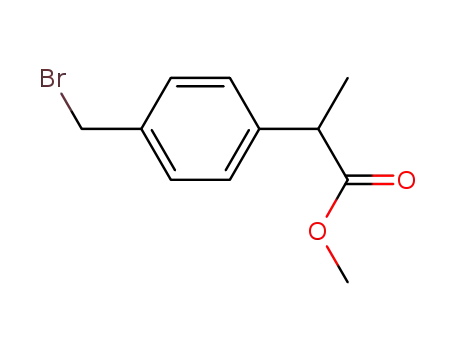
2-(4-bromomethylphenyl)propionic acid methyl ester
Relevant Products
-
2-Bromo-2-nitro-1,3-propanediol
CAS:52-51-7
-
AEO-7 Alcohol Ethoxylate
CAS:9002-92-0
-
AEO-3 Alcohol Ethoxylate
CAS:69011-36-5