111-87-5
- Product Name:Octanol
- Molecular Formula:C8H18O
- Purity:99%
- Molecular Weight:130.23
Product Details;
CasNo: 111-87-5
Molecular Formula: C8H18O
Appearance: colourless liquid
Perfect Factory Offer Excellent quality Octanol 111-87-5 with Safe Shipping
- Molecular Formula:C8H18O
- Molecular Weight:130.23
- Appearance/Colour:colourless liquid
- Melting Point:-15 °C(lit.)
- Refractive Index:n20/D 1.429(lit.)
- Boiling Point:194.665 °C at 760 mmHg
- PKA:15.27±0.10(Predicted)
- Flash Point:81.111 °C
- PSA:20.23000
- Density:0.824 g/cm3
- LogP:2.33920
1-Octanol(Cas 111-87-5) Usage
|
Safety Profile |
Poison by intravenous route.Moderately toxic by ingestion. Mutation data reported. Askin irritant. Combustible liquid when exposed to heat orflame; can react with oxidizing materials. To fight fire, usewater foam, fog, alcohol foam, dry chemical, CO2 |
InChI:InChI=1/C8H18O/c1-2-3-4-5-6-7-8-9/h9H,2-8H2,1H3
111-87-5 Relevant articles
Oxidation of zinc organometallics prepared by hydrozincation or carbozincation using oxygen
Klement, Ingo,Luetjens, Henning,Knochel, Paul
, p. 3161 - 3164 (1995)
Organozinc compounds prepared by the hyd...
Regioselective addition of stannylcyanocuprates to acetylenic ethers: A chemical and spectroscopic study
Cabezas,Oehlschlager
, p. 432 - 442 (1994)
The reactions of acetylenic ether 1 with...
Electrophilic Etherification of α-Heteroaryl Carbanions with Monoperoxyacetals as a Route to Ketene O, O- And N, O-Acetals
Paris, Timothy J.,Schwartz, Chris,Willand-Charnley, Rachel
, p. 2369 - 2384 (2021)
Alkyl ketene acetals are useful reactant...
Highly selective and stable ZnO-supported bimetallic RuSn catalyst for the hydrogenation of octanoic acid to octanol
Hidajat, Marcel Jonathan,Hwang, Dong-Won,Yun, Gwang-Nam
, (2021)
The chemoselective hydrogenation of biom...
Identification of a marine NADPH-dependent aldehyde reductase for chemoselective reduction of aldehydes
Li, Guangyue,Ren, Jie,Wu, Qiaqing,Feng, Jinhui,Zhu, Dunming,Ma, Yanhe
, p. 17 - 22 (2013)
A putative aldehyde reductase gene from ...
A facile zirconium(IV) chloride catalysed selective deprotection of t-butyldimethylsilyl (TBDMS) ethers
Sharma,Srinivas,Radha Krishna, Palakodety
, p. 4689 - 4691 (2003)
A simple and efficient protocol for the ...
Engineering carboxylic acid reductase for selective synthesis of medium-chain fatty alcohols in yeast
Hu, Yating,Zhu, Zhiwei,Gradischnig, David,Winkler, Margit,Nielsen, Jens,Siewers, Verena
, p. 22974 - 22983 (2020)
Medium-chain fatty alcohols (MCFOHs, C6 ...
Intrinsic isotope effects suggest that the reaction coordinate symmetry for the cytochrome P-450 catalyzed hydroxylation of octane is isozyme independent
Jones,Rettie,Trager
, p. 1242 - 1246 (1990)
The mechanism of the ω-hydroxylation of ...
Dod-S-Me and methyl 6-morpholinohexyl sulfide (MMS) as new odorless borane carriers
Patra, Pranab K.,Nishide, Kiyoharu,Fuji, Kaoru,Node, Manabu
, p. 1003 - 1006 (2004)
Odorless Dod-S-Me (1) and MMS (3) are de...
-
Gingras,Waters
, p. 3508,3511 (1954)
-
2-pyridylsilyl group as a multifunctional 'phase tag' for solution phase synthesis
Yoshida, Jun-ichi,Itami, Kenichiro,Mitsudo, Koichi,Suga, Seiji
, p. 3403 - 3406 (1999)
2-Pyridyldimethylsilyl (2-PyMe2Si) group...
Experimental study of chemical equilibria in the liquid-phase reaction between 1-octanol and ethanol to 1-ethoxyoctane
Guilera, Jordi,Ramirez, Eliana,Iborra, Montserrat,Tejero, Javier,Cunill, Fidel
, p. 2076 - 2082 (2013)
The equilibrium constants for the liquid...
Novel Cu and Cu2In/aluminosilicate type catalysts for the reduction of biomass-derived volatile fatty acids to alcohols
Harnos, Szabolcs,Onyestyak, Gyoergy,Barthos, Robert,Valyon, Jozsef,Stolcova, Magdalena,Kaszonyi, Alexander
, p. 1954 - 1962,9 (2012)
This work relates to the consecutive red...
REDUCTION OF ALDEHYDES AND KETONES WITH TETRAALKYLAMMONIUM BOROHYDRIDES
Raber, Douglas J.,Guida, Wayne C.,Shoenberger, Douglas C.
, p. 5107 - 5110 (1981)
Misinterpretations regarding the selecti...
Modulation of photodeprotection by the sunscreen protocol
Eivgi, Or,Levin, Efrat,Lemcoff, N. Gabriel
, p. 740 - 743 (2015)
A protocol for the selective photoremova...
In Vivo Reduction of Medium- to Long-Chain Fatty Acids by Carboxylic Acid Reductase (CAR) Enzymes: Limitations and Solutions
Horvat, Melissa,Winkler, Margit
, p. 5076 - 5090 (2020)
Fatty aldehyde production by chemical sy...
Ternary (Cu, Ni and Co) Nanocatalysts for Hydrogenation of Octanal to Octanol: An Insight into the Cooperative Effect
Valand, Jignesh,Dasireddy, Venkata D. B. C.,Singh, Sooboo,Friedrich, Holger B.
, p. 525 - 538 (2017)
Abstract: Ternary metal oxides (Cu–Ni–Co...
Pentaco-ordinate Silicon Compounds in Synthesis: Chemo- and Stereo-selective Reduction of Carbonyl Compounds using Trialkoxy-substituted Silanes and Alkali Metal Alkoxides
Hosomi, Akira,Hayashida, Hisashi,Kohra, Shinya,Tominaga, Yoshinori
, p. 1411 - 1412 (1986)
Carbonyl compounds are reduced with tria...
A Remarkable Inversion in the Selective Oxidation of Organoboranes and Thioethers
Brown, Herbert C.,Mandal, Arun K.
, p. 916 - 917 (1980)
-
Bacterial CYP153A monooxygenases for the synthesis of omega-hydroxylated fatty acids
Honda Malca, Sumire,Scheps, Daniel,Kuehnel, Lisa,Venegas-Venegas, Elena,Seifert, Alexander,Nestl, Bettina M.,Hauer, Bernhard
, p. 5115 - 5117 (2012)
CYP153A from Marinobacter aquaeolei has ...
Quantitative Evaluation of the Effect of the Hydrophobicity of the Environment Surrounding Br?nsted Acid Sites on Their Catalytic Activity for the Hydrolysis of Organic Molecules
Miura, Hiroki,Kameyama, Shutaro,Komori, Daiki,Shishido, Tetsuya
, p. 1636 - 1645 (2019)
Sulfo-functionalized siloxane gels with ...
Nickel catalyzed hydroboration with catecholborane
Kabalka,Narayana,Reddy
, p. 1019 - 1023 (1994)
Hydroborations of alkenes and alkyne wit...
Indium, as an efficient co-catalyst of Cu/Al2O3 in the selective hydrogenation of biomass derived fatty acids to alcohols
Onyestyák, Gy?rgy,Harnos, Szabolcs,Kalló, Dénes
, p. 19 - 24 (2012)
Octanoic acid (OA) as model reactant of ...
Deprotection of Acetals and Silyl Ethers Using Some ?-Acceptors
Tanemura, Kiyoshi,Suzuki, Tsuneo,Horaguchi, Takaaki
, p. 290 - 292 (1994)
Hydrolysis of dodecanol dimethyl acetal ...
A unique structural distribution pattern discovered for the cerebrosides from starfish Asterias amurensis
Yamaguchi, Ryosuke,Kanie, Yoshimi,Kanie, Osamu,Shimizu, Yoshitaka
, p. 115 - 122 (2019)
Cerebroside is an important family of th...
Effects of Organic Modifiers on a Palladium Catalyst in the Competitive Hydrogenation of 1-Octene Versus Octanal: An Evaluation of Solid Catalysts with an Ionic Liquid Layer
Miller, Stuart F.,Friedrich, Holger B.,Holzapfel, Cedric W.,Dasireddy, Venkata D. B. C.
, p. 2628 - 2636 (2015)
The competitive hydrogenation between 1-...
Hydroboration. 59. Thexylchloroborane-Methyl Sulfide. A New Stable Monohydroborating Agent with Exceptional Regioselectivity
Brown, Herbert C.,Sikorski, James A.,Kulkarni, Surendra U.,Lee, Hsiupu D.
, p. 863 - 872 (1982)
Under selected conditions, the hydrobora...
Highly active and selective platinum(0)-carbene complexes. Efficient, catalytic hydrosilylation of functionalised olefins
Marko, Istvan E.,Sterin, Sebastien,Buisine, Olivier,Berthon, Guillaume,Michaud, Guillaume,Tinant, Bernard,Declercq, Jean-Paul
, p. 1429 - 1434 (2004)
Readily available N-heterocyclic platinu...
OXIDATIVE CLEAVAGE OF SILICON-CARBON BOND WITH TRIMETHYLAMINE-N-OXIDE. NEW ACCESS TO PRIMARY ALCOHOLS AND ALDEHYDES FROM TERMINAL ALKENES AND ALKYNES
Sakurai, Hideki,Ando, Masatomo,Kawada, Nobuo,Sato, Kazuhiko,Hosomi, Akira
, p. 75 - 76 (1986)
Oxidative cleavage of carbon-silicon bon...
Improving the catalytic behavior of Ni/Al2O3 by indium in reduction of carboxylic acid to alcohol
Onyestyák, Gy?rgy,Harnos, Szabolcs,Kalló, Dénes
, p. 184 - 188 (2011)
Octanoic acid (OA) was used as reactant ...
Regulation of Iron-Catalyzed Olefin Hydroboration by Ligand Modifications at a Remote Site
Tseng, Kuei-Nin T.,Kampf, Jeff W.,Szymczak, Nathaniel K.
, p. 411 - 415 (2015)
An amide-derived N,N,N-Fe(II) complex ca...
Alkyne [2 + 2 + 2] Cyclotrimerization Catalyzed by a Low-Valent Titanium Reagent Derived from CpTiX3 (X = Cl, O- i-Pr), Me3SiCl, and Mg or Zn
Okamoto, Sentaro,Yamada, Takeshi,Tanabe, Yu-Ki,Sakai, Masaki
, p. 4431 - 4438 (2018)
Inter-, partially intra-, and intramolec...
Amides as Nucleophiles: Reaction of Alkyl Halides with Amides or with Amides and Water. A New Look at an Old Reaction
Brace, Neal O.
, p. 1804 - 1811 (1993)
Heating of formamide with an alkyl halid...
An efficient oxidative cleavage of carbon-silicon bonds by a dioxygen/hydroquinone system
Tamao,Hayashi,Ito
, p. 6533 - 6536 (1989)
Carbon-silicon bonds in alkylalkoxysilan...
Thexylchloroborane. A Versatile Reagent for the Preparation of Mixed Thexyldiorganoboranes
Zweifel, George,Pearson, Norman R.
, p. 5919 - 5920 (1980)
-
H2-driven biotransformation of n-octane to 1-octanol by a recombinant Pseudomonas putida strain co-synthesizing an O2-tolerant hydrogenase and a P450 monooxygenase
Lonsdale, Thomas H.,Lauterbach, Lars,Honda Malca, Sumire,Nestl, Bettina M.,Hauer, Bernhard,Lenz, Oliver
, p. 16173 - 16175 (2015)
An in vivo biotransformation system is p...
Unveiling the dual role of the cholinium hexanoate ionic liquid as solvent and catalyst in suberin depolymerisation
Ferreira, Rui,Garcia, Helga,Sousa, Andreia F.,Guerreiro, Marina,Duarte, Filipe J. S.,Freire, Carmen S. R.,Calhorda, Maria Jose,Silvestre, Armando J. D.,Kunz, Werner,Rebelo, Luis Paulo N.,Silva Pereira, Cristina
, p. 2993 - 3002 (2014)
Disruption of the three-dimensional netw...
Influence of sorption to dissolved humic substances on transformation reactions of hydrophobic organic compounds in water. Part II: Hydrolysis reactions
Georgi, Anett,Trommler, Ulf,Reichl, Annett,Kopinke, Frank-Dieter
, p. 1452 - 1460 (2008)
The effect of dissolved humic acid (HA) ...
MOF-derived Cu@C catalyst for the liquid-phase hydrogenation of esters
Zhao, Yujun,Wu, Xiaoqian,Zhou, Jiahua,Wang, Yue,Wang, Shengping,Ma, Xinbin
, p. 883 - 886 (2018)
MOF derived core-shell Cu@C was prepared...
Mild and efficient tetrahydropyranylation and deprotection of alcohols catalyzed by heteropoly acids
Molnar, Arpad,Beregszaszi, Timea
, p. 8597 - 8600 (1996)
A simple, mild and effective method to f...
Electrocatalytic hydrogenation of octyl aldehyde over Pd catalysts
Cirtiu, Ciprian M.,Menard, Hugues
, p. 475 - 478 (2007)
The electrocatalytic hydrogenation (ECH)...
Hydrolysis of Water-insoluble Esters by Octadecyl Immobilized H-ZSM-5 Catalyst in a Water-Toluene System
Ogawa, Haruo,Tensai, Koh,Taya, Kazuo,Chihara, Teiji
, p. 1246 - 1247 (1990)
In the hydrolysis of water-insoluble est...
A Remarkably Simple Class of Imidazolium-Based Lipids and Their Biological Properties
Wang, Da,Richter, Christian,Rühling, Andreas,Drücker, Patrick,Siegmund, Daniel,Metzler-Nolte, Nils,Glorius, Frank,Galla, Hans-Joachim
, p. 15123 - 15126 (2015)
A series of imidazolium salts bearing tw...
Selective Electroenzymatic Oxyfunctionalization by Alkane Monooxygenase in a Biofuel Cell
Abdellaoui, Sofiene,Chen, Hui,Kummer, Matthew J.,Malapit, Christian A.,Minteer, Shelley D.,You, Chun,Yuan, Mengwei
, p. 8969 - 8973 (2020)
Aliphatic synthetic intermediates with h...
Novel hydroborating agents from Silylamine-boranes
Soderquist, John A.,Medina, Jesus R.,Huertas, Ramon
, p. 6119 - 6122 (1998)
Exhibiting a broad spectrum of hydrobora...
Asymmetric transformation of enones with Synechococcus sp. PCC 7942
Shimoda, Kei,Kubota, Naoji,Hamada, Hiroki,Yamane, Shin-Ya,Hirata, Toshifumi
, p. 2269 - 2272 (2004)
Asymmetric transformation of enones was ...
Regiodivergent Reductive Opening of Epoxides by Catalytic Hydrogenation Promoted by a (Cyclopentadienone)iron Complex
De Vries, Johannes G.,Gandini, Tommaso,Gennari, Cesare,Jiao, Haijun,Pignataro, Luca,Stadler, Bernhard M.,Tadiello, Laura,Tin, Sergey
, p. 235 - 246 (2022/01/03)
The reductive opening of epoxides repres...
BiCl3-Facilitated removal of methoxymethyl-ether/ester derivatives and DFT study of -O-C-O- bond cleavage
Pacherille, Angela,Tuga, Beza,Hallooman, Dhanashree,Dos Reis, Isaac,Vermette, Mélodie,Issack, Bilkiss B.,Rhyman, Lydia,Ramasami, Ponnadurai,Sunasee, Rajesh
supporting information, p. 7109 - 7116 (2021/05/03)
A simple method for the cleavage of meth...
Radical Chain Reduction via Carbon Dioxide Radical Anion (CO2?-)
Hendy, Cecilia M.,Jui, Nathan T.,Lian, Tianquan,Smith, Gavin C.,Xu, Zihao
supporting information, p. 8987 - 8992 (2021/07/01)
We developed an effective method for red...
KB3H8: An environment-friendly reagent for the selective reduction of aldehydes and ketones to alcohols
Li, Xinying,Mi, Tongge,Guo, Wenjing,Ruan, Zhongrui,Guo, Yu,Ma, Yan-Na,Chen, Xuenian
supporting information, p. 12776 - 12779 (2021/12/10)
Selective reduction of aldehydes and ket...
111-87-5 Process route
-
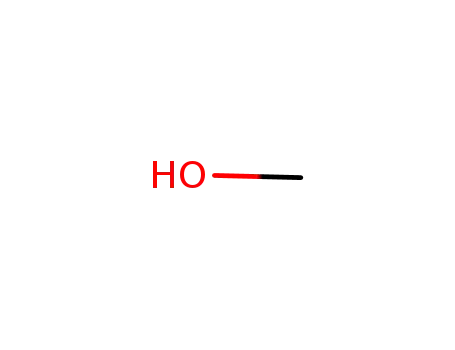
- 67-56-1
methanol

-

- 629-82-3
di-n-octyl ether

-
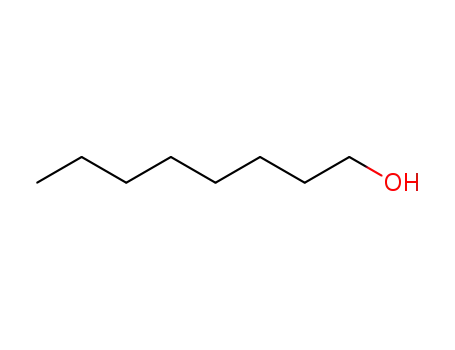
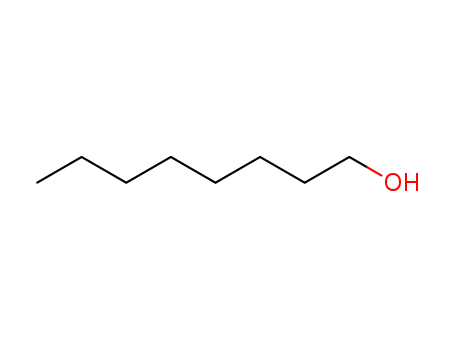
- 111-87-5
octanol

-
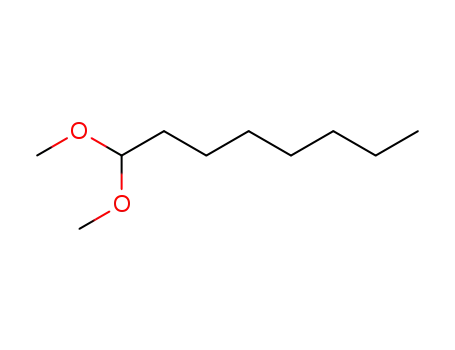
- 10022-28-3
1,1-dimethoxyoctane
| Conditions | Yield |
|---|---|
|
In acetic acid; for 5.4h; Yield given; electrolysis: platinum-plate anode, carbon-rod cathode, Bu4NBF4, 20 F/mol, 0.5 A, 20 V;
|
-
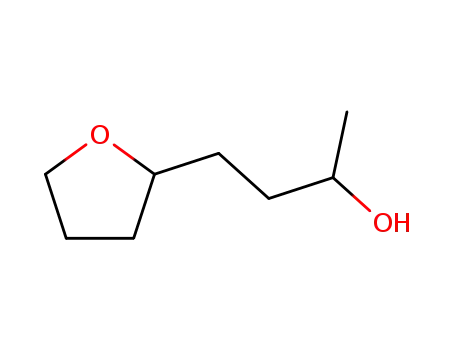
- 3208-43-3,4527-76-8
4-tetrahydrofuran-2-yl-butan-2-ol

-
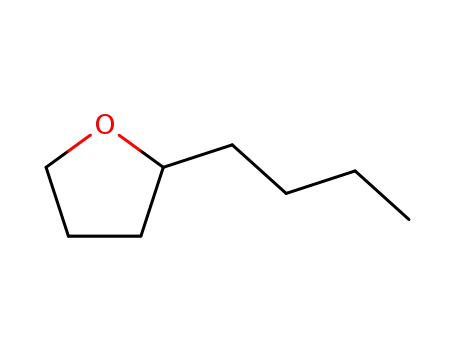
- 1004-29-1
2-butanyltetrahydrofuran

-

- 111-87-5
octanol

-

- 629-82-3
di-n-octyl ether
| Conditions | Yield |
|---|---|
|
With ruthenium-carbon composite; 1-(4-butylcarboxylic acid)-3-(n-butyl)-imidazolium bis(trifluoromethylsulfonyl)imide; hydrogen; at 150 ℃; for 15h; under 90009 Torr; Reagent/catalyst; Ionic liquid;
|
58.6% 27.1% 9.1% |
|
With 1-(4-butylcarboxylic acid)-3-(n-butyl)-imidazolium bis(trifluoromethylsulfonyl)imide; hydrogen; at 150 ℃; for 15h; under 90009 Torr; Reagent/catalyst; Ionic liquid;
|
25.8% 44.9% 26.1% |
|
With N,N,N-tributyl-1-(4-butylsulfonic acid)-aminium bis(trifluoromethylsulfonyl)imide; hydrogen; at 150 ℃; for 15h; under 90009 Torr; Reagent/catalyst; Ionic liquid;
|
11.7% 41.5% 34.5% |
|
With 5 wt% ruthenium/carbon; hydrogen; at 150 ℃; for 15h; under 90009 Torr; Ionic liquid;
|
111-87-5 Upstream products
-
75-21-8
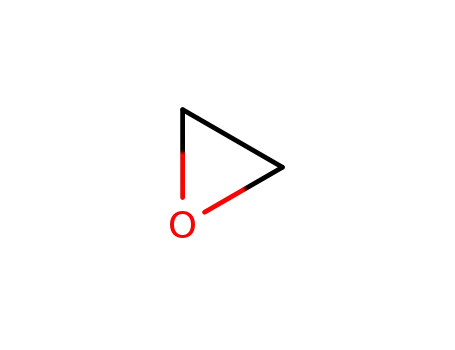
oxirane
-
3761-92-0
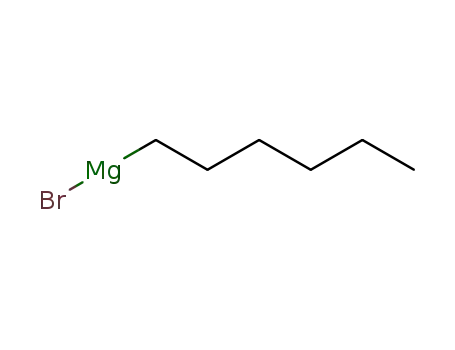
n-hexylmagnesium bromide
-
123-91-1
1,4-dioxane
-
60-29-7
diethyl ether
111-87-5 Downstream products
-
10020-43-6
2-octyloxyethanol
-
929-56-6
1-methoxyoctane
-
123080-90-0
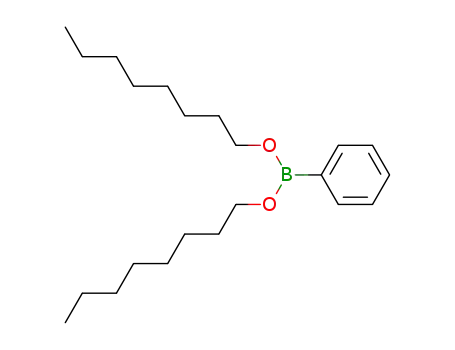
C6H5B(O-n-C8H17)2
-
16436-00-3
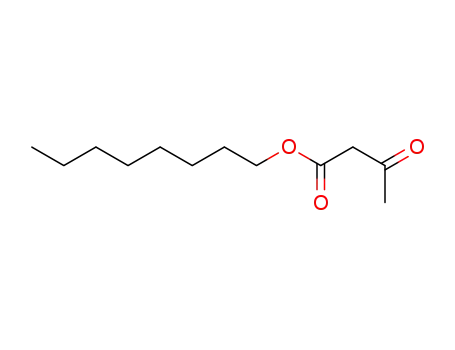
octyl 3-oxobutanoate
Relevant Products
-
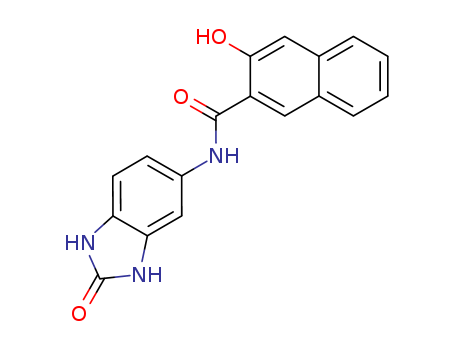
5-(2′-Hydroxy-3′-Naphthamide)-2-Benzimidazolone
CAS:26848-40-8
-
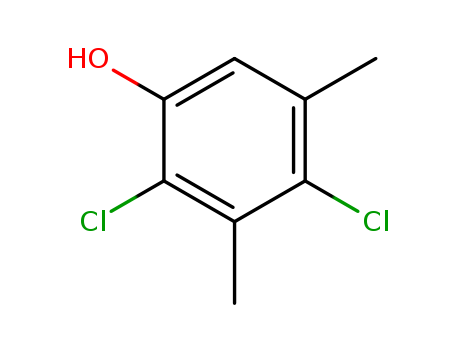
2,4-Dichloro-3,5-Dimethyl-Phenol
CAS:133-53-9
-
AEO-9
CAS:68439-45-2