112-30-1
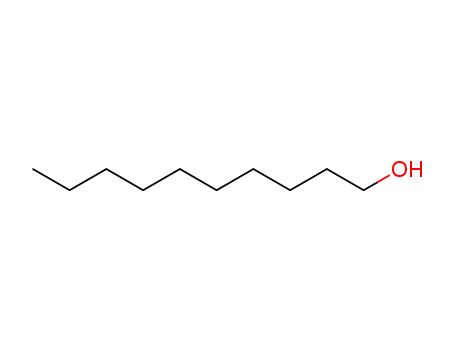
- Product Name:Decanol
- Molecular Formula:C10H22O
- Purity:99%
- Molecular Weight:158.284
Product Details;
CasNo: 112-30-1
Molecular Formula: C10H22O
Appearance: colorless viscous liquid
Buy Good Quality Decanol 112-30-1 with a minimum purity of 99%
- Molecular Formula:C10H22O
- Molecular Weight:158.284
- Appearance/Colour:colorless viscous liquid
- Vapor Pressure:1 mm Hg ( 70 °C)
- Melting Point:6 °C
- Refractive Index:n20/D 1.437(lit.)
- Boiling Point:227.8 °C at 760 mmHg
- PKA:15.21±0.10(Predicted)
- Flash Point:108 °C
- PSA:20.23000
- Density:0.8297 g/cm3
- LogP:3.11940
Decyl alcohol(Cas 112-30-1) Usage
|
description |
1-Decanol, also known as decyl alcohol or n-decyl alcohol, is a straight chain fatty alcohol with ten carbon atoms and the molecular formula CH3(CH2)9OH. It is a colorless viscous liquid that is insoluble in water. It is colorless and has a strong odor. It is a clear colorless liquid with a sweet fat-like odor. It has a flash point of 180°F. It is less dense than water and insoluble in water. Its vapors are heavier than air. |
|
Applications |
Decyl alcohol can be used for the production of plasticizers, lubricants, surfactants and solvents. It is also used to study the thermal properties of polymer-monolithic stationary phases. Furthermore, it can used to enhance homomeric glycine receptor function. In addition to this, it is used in daily flavor, food flavor and cosmetics. In addition, the 5HT2α receptors were inhibited by the presence of decyl alcohol. This action has many psychological repercussions on an individual. It can also be used as a plant growth regulator of tobacco. |
|
Warning and Risk |
Decyl alcohol causes a high irritability to skin and eyes, when splashed into the eyes it can cause permanent damage. Also inhalation and ingestion can be harmful, it can also function as a narcotic. It is also harmful in the environment. |
|
Definition |
ChEBI: 1-Decanol is a fatty alcohol consisting of a hydroxy function at C-1 of an unbranched saturated chain of ten carbon atoms. It has a role as a metabolite and a protic solvent. It is a primary alcohol and a fatty alcohol. |
|
Production Methods |
1-Decanol is prepared commercially by sodium reduction or by the high-pressure catalytic reduction of coconut oil, coconut fatty acids, or esters . It is also produced by the Ziegler process, which involves oxidation of trialkylaluminum compounds. |
|
Preparation |
By sodium reduction or high-pressure catalytic hydrogenation of the esters of naturally occurring capric acid, or by oligomerization of ethylene using aluminium alkyl technology. |
|
Application |
decyl alcohol can be used for any number of functions, including as an emollient, a foam-booster, a surfactant and a viscosity controller, as well to mask odor and as a fixative in perfumes. Decyl alcohol occurs naturally in sweet orange and ambrette seed. It is also derived commercially from liquid paraffin. |
|
Aroma threshold values |
Detection: 6 to 47 ppb |
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 38, p. 3223, 1973 DOI: 10.1021/jo00958a031Synthetic Communications, 12, p. 287, 1982 DOI: 10.1080/00397918209409235 |
|
General Description |
A clear colorless liquid with a sweet fat-like odor. Flash point 180°F. Less dense than water and insoluble in water. Vapors are heavier than air. |
|
Air & Water Reactions |
Insoluble in water. |
|
Reactivity Profile |
Decyl alcohol attacks plastics. REF [Handling Chemicals Safely, 1980. p. 236]. Acetyl bromide reacts violently with alcohols or water, [Merck 11th ed., 1989]. Mixtures of alcohols with concentrated sulfuric acid and strong hydrogen peroxide can cause explosions. Example: An explosion will occur if dimethylbenzylcarbinol is added to 90% hydrogen peroxide then acidified with concentrated sulfuric acid. Mixtures of ethyl alcohol with concentrated hydrogen peroxide form powerful explosives. Mixtures of hydrogen peroxide and 1-phenyl-2-methyl propyl alcohol tend to explode if acidified with 70% sulfuric acid, [Chem. Eng. News 45(43):73(1967); J, Org. Chem. 28:1893(1963)]. Alkyl hypochlorites are violently explosive. They are readily obtained by reacting hypochlorous acid and alcohols either in aqueous solution or mixed aqueous-carbon tetrachloride solutions. Chlorine plus alcohols would similarly yield alkyl hypochlorites. They decompose in the cold and explode on exposure to sunlight or heat. Tertiary hypochlorites are less unstable than secondary or primary hypochlorites, [NFPA 491 M, 1991]. Base-catalysed reactions of isocyanates with alcohols should be carried out in inert solvents. Such reactions in the absence of solvents often occur with explosive violence, [Wischmeyer(1969)]. |
|
Health Hazard |
Direct contact can produce eye irritation; low general toxicity. |
|
Fire Hazard |
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water. |
|
Chemical Reactivity |
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent. |
|
Safety Profile |
Moderately toxic by skin contact. Wdly toxic by ingestion and inhalation. A severe human skin and eye irritant. Experimental reproductive effects. Questionable carcinogen with experimental tumorigenic data. Combustible when exposed to heat or flame; can react with oxidzing materials. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes. See also ALCOHOLS. |
|
Synthesis |
Synthetically prepared from coconut oil derivatives; by reduction of some capric esters, such as methyl caprate. |
|
Carcinogenicity |
1-Decanol showed weak to moderate tumor-promoting activity when applied three times a week for 60 weeks to the skin of female Swiss mice that previously received an initiating dose of dimethylbenz[ a]anthracene . |
|
Metabolism |
See alcohol C-8. |
|
Purification Methods |
Fractionally distil n-decanol in an all-glass unit at 10mm pressure (b 110o), then fractionally crystallise by partial freezing. Also purify by preparative GLC, and by passage through alumina before use. [Beilstein 1 IV 1815.] |
InChI:InChI=1/C16H38N2.C2H6O.2BrH/c1-17(2,3)15-13-11-9-7-8-10-12-14-16-18(4,5)6;1-2-3;;/h7-16H2,1-6H3;3H,2H2,1H3;2*1H/q+2;;;/p-2
112-30-1 Relevant articles
Process Development for the Rhodium-Catalyzed Reductive Amination in a Thermomorphic Multiphase System
Künnemann, Kai U.,Bianga, Jonas,Scheel, Ricarda,Seidensticker, Thomas,Dreimann, Jens M.,Vogt, Dieter
, p. 41 - 49 (2020)
For the first time, the successful appli...
Reduction of Carboxylic Acid with 2-Propanol over Zirconia-Titania
Takahashi, Kyoko,Shibagaki, Makoto,Kuno, Hideyuki,Matsushita, Hajime
, p. 839 - 840 (1993)
The reduction of long carbon chain carbo...
Reduction of aldehydes using trialkylboranes in ionic liquids
Kabalka,Malladi
, p. 2191 - 2191 (2000)
Non-aqueous ionic liquids, molten salts,...
Oxidation of sterically hindered alkoxysilanes and phenylsilanes under basic conditions
Smitrovich, Jacqueline H.,Woerpel
, p. 6044 - 6046 (1996)
-
Deprotection of mono and dimethoxy phenyl methyl ethers using catalytic amounts of DDQ
Chandrasekhar,Sumithra,Yadav
, p. 1645 - 1646 (1996)
4-Methoxy and 3,4 dimethoxy benzyl ether...
Selective Removal of the Methyldiphenylsilyl Protecting Group Using Azide Ion
Monger, Steven J.,Parry, David M.,Roberts, Stanley M.
, p. 381 - 382 (1989)
Sodium azide in dimethylformamide effect...
Hydrogen transfer hydrozirconation of alkenes with iBuZrCp2Cl catalyzed by Lewis-acidic metal compounds containing Al, Zn, Si, Ag, and Pd
Makabe, Hidefumi,Negishi, Ei-Ichi
, p. 969 - 971 (1999)
The hydrozirconation reaction of monosub...
Structure sensitivity in the ruthenium nanoparticle catalyzed aqueous-phase Fischer-Tropsch reaction
Quek, Xian-Yang,Pestman, Robert,Van Santen, Rutger A.,Hensen, Emiel J. M.
, p. 3510 - 3523 (2014)
Low-temperature Fischer-Tropsch reaction...
Convenient method for the preparation of catecholborane and promotion of the formation of alkenyl catecholborane using BH3 complexes
Suseela, Y.,Periasamy, M.
, p. 47 - 52 (1993)
Catecholborane is prepared in benzene by...
Deprotection of benzyl ethers using 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ) under photoirradiation
Rahim, Mohammad Abdur,Matsumura, Shuichi,Toshima, Kazunobu
, p. 7307 - 7309 (2005)
The deprotection of benzyl ethers was ef...
-
Thaisrivongs,S.,Wuest,J.D.
, p. 3243 - 3247 (1977)
-
Reactions of Amino Acid Decyl Esters with Nucleophiles Catalyzed by Polymer-Supported Amine-Metal Complexes
Ohtani, Noritaka,Inoue, Yukihiko,Inagaki, Yuichi,Fukuda, Kenji,Nishiyama, Taisuke
, p. 1669 - 1676 (1995)
The reactions of alanine decyl ester (Al...
Unexpected deprotection of silyl and THP ethers induced by serious disparity in the quality of Pd/C catalysts and elucidation of the mechanism
Ikawa, Takashi,Sajiki, Hironao,Hirota, Kosaku
, p. 6189 - 6195 (2004)
Commercial Pd/C catalysts show different...
THERMAL STABILITY IN RELATION TO HYDROLYSIS OF SODIUM DECYLSULPHATE IN A SOLUTION WITH NEMATIC LYOTROPIC PROPERTIES.
Hochapfel,Boidart,Laurent
, p. 201 - 209 (1981)
Studied the effect of temperature as a f...
Transfer hydrogenation of aromatic and linear aldehydes catalyzed using Cp*Ir(pyridinesulfonamide)Cl complexes under base-free conditions
Townsend, Tanya M.,Kirby, Christopher,Ruff, Andrew,O'Connor, Abby R.
, p. 7 - 13 (2017)
Cp*Ir(pyridinesulfonamide)Cl (Cp*?=?pent...
Novel deprotection of SEM ethers: A very mild and selective method using magnesium bromide
Vakalopoulos, Alexandros,Hoffmann
, p. 1447 - 1450 (2000)
(Matrix presented) New lability and stab...
A mild, efficient, and selective method for the desilylation of more common trialkylsilyl ethers by cerium(III) chloride heptahydrate and sodium iodide in acetonitrile
Bartoli, Giuseppe,Bosco, Marcella,Marcantoni, Enrico,Sambri, Letizia,Torregiani, Elisabetta
, p. 209 - 211 (1998)
Treatment of trialkylsilyl ethers with c...
Hydrogenation versus hydrogenolysis with a safe, selective and reusable catalyst: Palladium black on Teflon
Belotti, Damien,Cantagrel, Guillaume,Combellas, Catherine,Cossy, Janine,Kanoufi, Frederic,Nunige, Sandra
, p. 761 - 764 (2005)
Palladium black deposit is obtained by r...
Cleavage of the THP protecting group under Pd/C-catalyzed hydrogenation conditions
Kaisalo, Leena,Hase, Tapio
, p. 7699 - 7701 (2001)
Alcohol and phenol THP or ethoxyethyl et...
Reduction of Aldehydes and Ketones Using Sodium Formate in 1-Methyl-2-pyrrolidinone
Babler, James H.,Sarussi, Steven J.
, p. 3367 - 3369 (1981)
-
Significant supplier-dependent disparity in catalyst activity of commercial Pd/C toward the cleavage of triethylsilyl ether
Sajiki, Hironao,Ikawa, Takashi,Hirota, Kosaku
, p. 7407 - 7410 (2003)
Pd/C catalysts exhibit remarkable suppli...
Transition metal catalysis in fluorous media: Practical application of a new immobilization principle to rhodium-catalyzed hydroborations of alkenes and alkynes
Juliette, Jerrick J. J.,Rutherford, Drew,Horváth, István T.,Gladysz
, p. 2696 - 2704 (1999)
Addition of a yellow-orange toluene solu...
Copper(II) chloride dihydrate: A catalytic agent for the deprotection of tetrahydropyranyl ethers (THP ethers) and 1-ethoxyethyl ethers (EE ethers)
Wang, Jianbo,Zhang, Chao,Qu, Zhaohui,Hou, Yihua,Chen, Bei,Wu, Peng
, p. 294 - 295 (1999)
Tetrahydropyranyl ethers (THP groups) an...
An Immobilized Organotin Catalyst for Reduction of Ketones and Aldehydes
Matlin, Stephen A.,Gandham, Permjit S.
, p. 798 - 799 (1984)
A dialkyltin dimethoxide linked to the s...
Selective and unprecedented oxidative deprotection of allyl ethers with DDQ
Yadav,Chandrasekhar,Sumithra,Kache, Rajashaker
, p. 6603 - 6606 (1996)
-
Preparation of samarium(II) iodide: Quantitative evaluation of the effect of water, oxygen, and peroxide content, preparative methods, and the activation of samarium metal
Szostak, Michal,Spain, Malcolm,Procter, David J.
, p. 3049 - 3059 (2012)
Samarium(II) iodide (SmI2) is one of the...
REACTIONS OF ALLYLIC CARBONATES CATALYZED BY PALLADIUM, RHODIUM, RUTHENIUM, MOLYBDENUM, AND NICKEL COMPLEXES; ALLYLATION OF CARBONUCLEOPHILES AND DECARBOXYLATION-DEHYRDOGENATION
Minami, Ichiro,Shimizu, Isao,Tsuji, Jiro
, p. 269 - 280 (1985)
Allylation of carbonucleophiles with all...
Hydrogen-transfer reduction of carbonyl compounds promoted by nickel nanoparticles
Alonso, Francisco,Riente, Paola,Yus, Miguel
, p. 1847 - 1852 (2008)
Nickel(0) nanoparticles, generated from ...
Selective Production of Linear Aldehydes and Alcohols from Alkenes using Formic Acid as Syngas Surrogate
Chen, Junjun,Hua, Kaimin,Liu, Xiaofang,Deng, Yuchao,Wei, Baiyin,Wang, Hui,Sun, Yuhan
, p. 9919 - 9924 (2021/05/31)
Performing carbonylation without the use...
Synthesis and surface-active properties of novel cleavable gemini surfactants
Gilbert, Elangeni Ana,Guastavino, Javier Fernando,Murguía, Marcelo César
, (2021/09/14)
A novel series of quaternary ammonium ge...
Deoxygenative hydroboration of primary, secondary, and tertiary amides: Catalyst-free synthesis of various substituted amines
An, Duk Keun,Jaladi, Ashok Kumar,Kim, Hyun Tae,Yi, Jaeeun
, (2021/11/17)
Transformation of relatively less reacti...
Enantiomeric synthesis of natural alkylglycerols and their antibacterial and antibiofilm activities
Fernández Montoya, Deicy J.,Contreras Jordan, Luis A.,Moreno-Murillo, Bárbara,Silva-Gómez, Edelberto,Mayorga-Wandurraga, Humberto
supporting information, p. 2544 - 2550 (2019/11/13)
Alkylglycerols (AKGs) are bioactive natu...
112-30-1 Process route
-
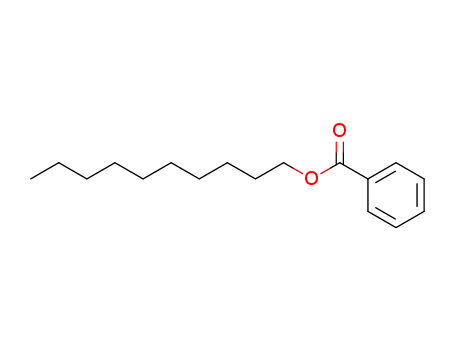
- 36685-97-9
n-decyl benzoate

-
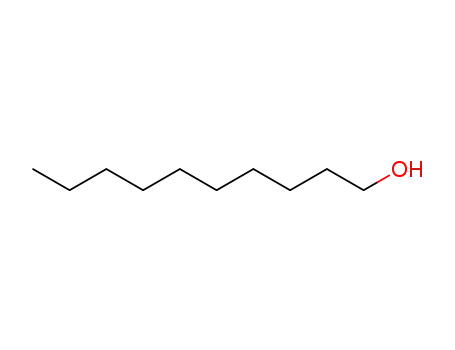
- 112-30-1
1-Decanol

-
- 100-51-6,185532-71-2
benzyl alcohol
| Conditions | Yield |
|---|---|
|
With lithium-
|
66% 67% |
-
- 64-17-5
ethanol

-
- 60-29-7,927820-24-4
diethyl ether

-
- 111-87-5
octanol

-
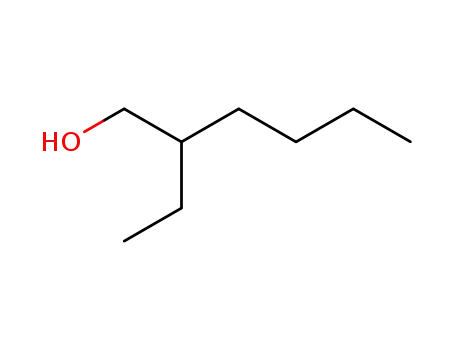
- 104-76-7
2-Ethylhexyl alcohol

-
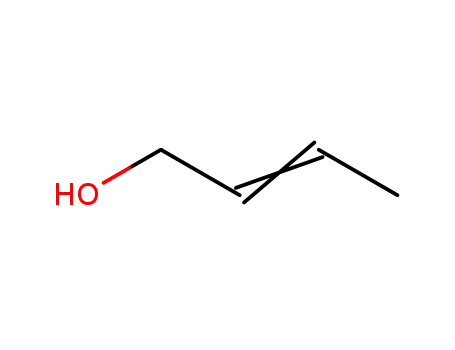
- 6117-91-5,542-72-3
(E/Z)-2-buten-1-ol

-
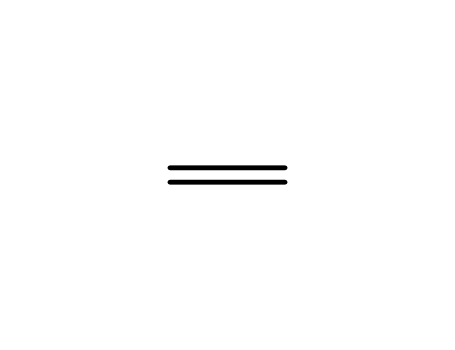
- 74-85-1
ethene

-
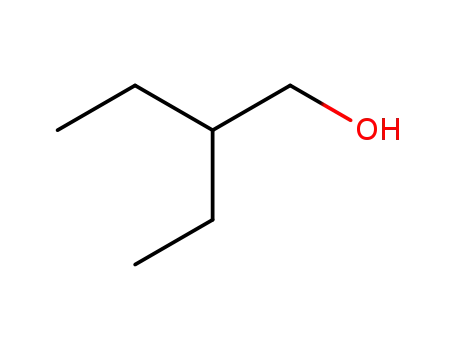
- 97-95-0
2-ethyl-1-butanol

-

- 112-30-1
1-Decanol

-
- 75-07-0,9002-91-9
acetaldehyde

-
- 105-54-4
butanoic acid ethyl ester

-
- 106-99-0,130983-70-9,29406-96-0,9003-17-2
buta-1,3-diene

-
- 71-36-3
butan-1-ol

-
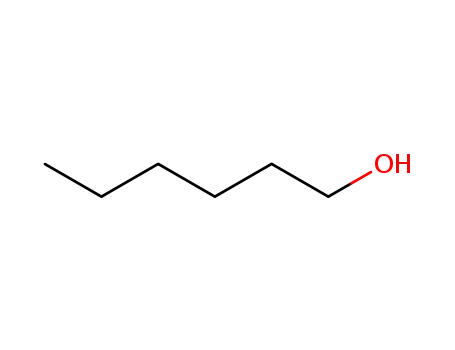
- 111-27-3
hexan-1-ol
| Conditions | Yield |
|---|---|
|
With deficient carbonate-containing hydroxyapatites (HapD); at 300 - 400 ℃; Reagent/catalyst; Temperature; Overall yield = 14 %; Catalytic behavior; Inert atmosphere;
|
112-30-1 Upstream products
-
75-21-8
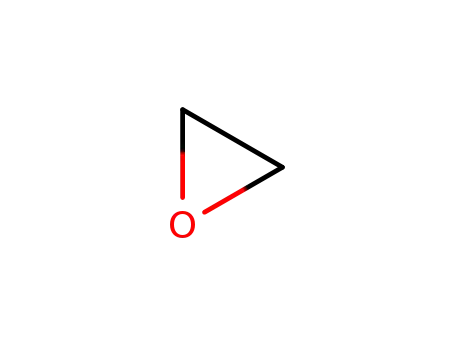
oxirane
-
17049-49-9
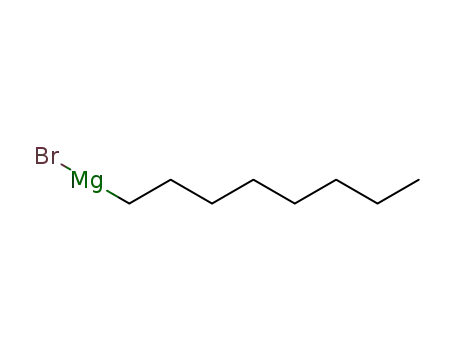
octylmagnesium bromide
-
2404-44-6
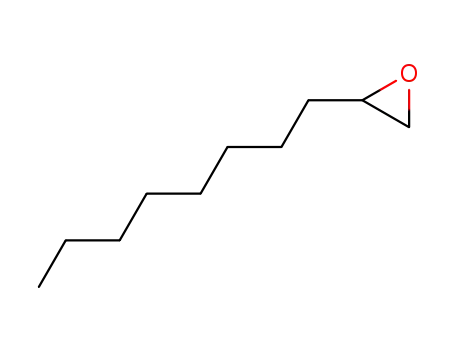
1,2-Epoxydecane
-
50-00-0
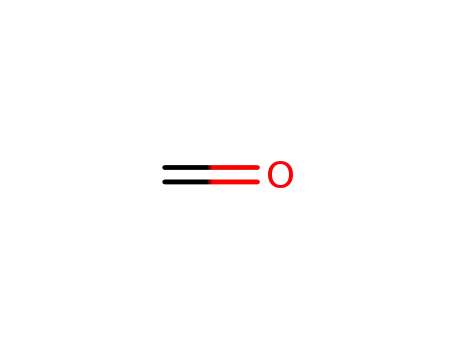
formaldehyd
112-30-1 Downstream products
-
5335-70-6
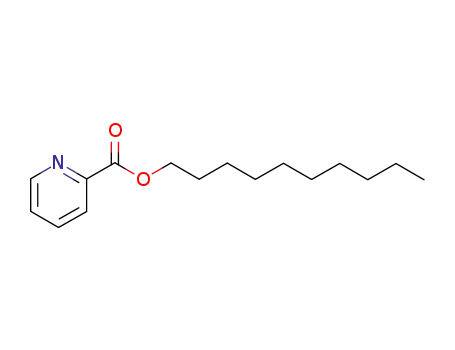
pyridine-2-carboxylic acid decyl ester
-
55488-51-2
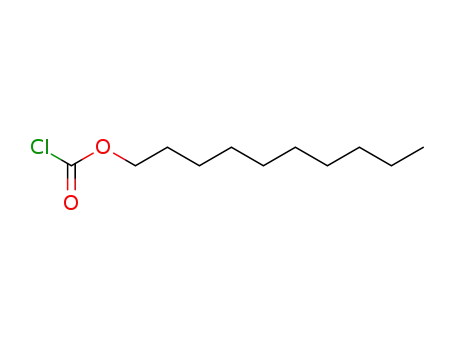
n-decyl chloroformate
-
24539-60-4
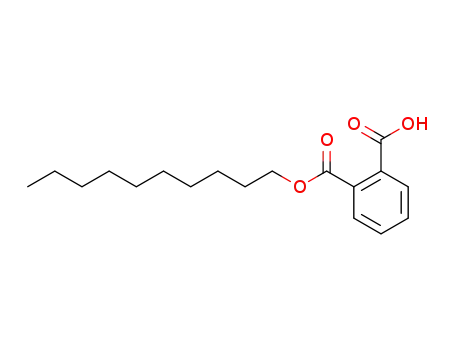
mono-n-decyl phthalate
-
18402-10-3
decyloxytrimethylsilane
Relevant Products
-
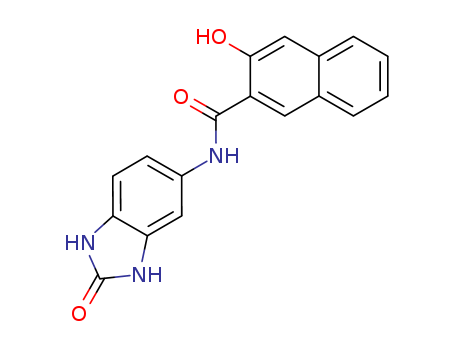
5-(2′-Hydroxy-3′-Naphthamide)-2-Benzimidazolone
CAS:26848-40-8
-
Povidone Iodine
CAS:25655-41-8
-
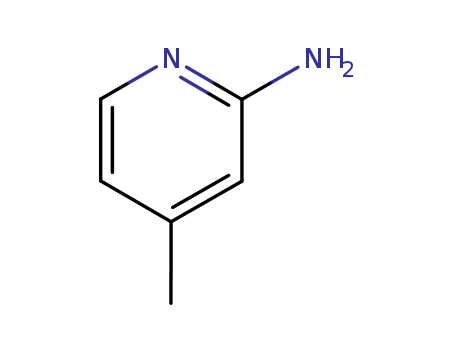
2-Amino-4-Methylpyridine
CAS:695-34-1