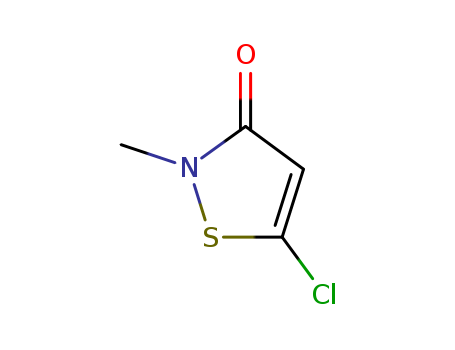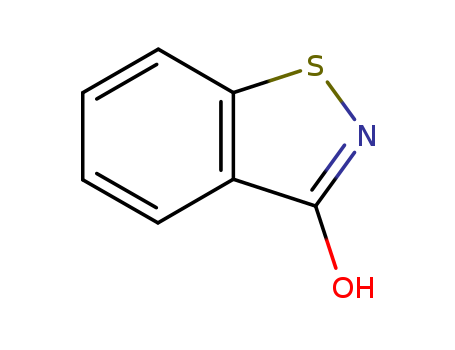
2634-33-5
- Product Name:1,2-benzeisothiazoline-3-one
- Molecular Formula:C7H5NOS
- Purity:99%
- Molecular Weight:151.189
Product Details;
CasNo: 2634-33-5
Molecular Formula: C7H5NOS
Appearance: white to pale yellow powder
Manufacturer supply high quality 1,2-benzeisothiazoline-3-one 2634-33-5 with GMP standards
- Molecular Formula:C7H5NOS
- Molecular Weight:151.189
- Appearance/Colour:white to pale yellow powder
- Vapor Pressure:0.183mmHg at 25°C
- Melting Point:154-158 ºC
- Refractive Index:1.66
- Boiling Point:204.5 ºC at 760 mmHg
- PKA:10.19±0.20(Predicted)
- Flash Point:77.5 ºC
- PSA:61.10000
- Density:1.367 g/cm3
- LogP:1.58960
1,2-Benzisothiazolin-3-one(Cas 2634-33-5) Usage
|
Application |
1,2-benzisothiazol-3(2H)-one (BIT) has been widely used in high concentrations for microbial growth control in many domestic and industrial processes, its potential eco-risk should be assessed. |
|
Definition |
ChEBI: An organic heterobicyclic compound based on a fused 1,2-thiazole and benzene bicyclic ring skeleton, with the S atom positioned adjacent to one of the positions of ring fusion. |
|
General Description |
1,2-Benzisothiazol-3(2H)-one is a commonly used biocide in industrial and consumer products, which possesses antimicrobial activity against gram positive and gram negative bacteria. It is mainly used in packaging, adhesives, detergents, disinfectants, sunscreen lotions, paints and lubricants. |
|
Flammability and Explosibility |
Notclassified |
|
Contact allergens |
BIT, both an irritant and a skin sensitizer, is widely used in industry as a preservative in water-based solutions such as pastes, paints, and cutting oils. Occupational dermatitis has been reported mainly due to cutting fluids and greases, in paint manufacturers, pottery moldmakers, acrylic emulsions manufacturers, plumber,printers and lithoprinters, paper makers, analytical laboratory, rubber factory, and employees manufacturing air fresheners. It is also a preservative in vinyl gloves. |
InChI:InChI=1/C7H5NOS/c9-7-5-3-1-2-4-6(5)10-8-7/h1-4H,(H,8,9)
2634-33-5 Relevant articles
Novel palladium(II) and platinum(II) complexes of biocidal benzisothiazolinone (Bit); X-ray crystal structures of co-crystallised Bit/BitO and cis-Pd(en)(Bit-1H)2·H2O
Griffith, Darren M.,Haughey, Aisleen,Chahal, Sunisha,Müller-Bunz, Helge,Marmion, Celine J.
, p. 2333 - 2337 (2010)
Reaction of benzisothiazolinone (Bit), a...
Process Development of 1,2-Benzisothiazolin-3(2H)-one by Replacing of the Toxic Materials
Jin, Chun Keun,Moon, Jung-Kyen,Lee, Woo Song,Nam, Keun Soo
, p. 1967 - 1968 (2003)
1,2-Benzisothiazolin-3(2H)-one (2) was s...
Dimer impurity in ziprasidone hydrochloride raw material and preparation method thereof
-
Paragraph 0133-0136, (2021/06/09)
The invention provides a dimer impurity ...
Synthesis method of 1, 2-benzisothiazoline-3-ketone
-
Paragraph 0047-0054, (2021/04/10)
The invention discloses a synthesis meth...
Method for preparing 1, 2-benzisothiazolin-3-one through catalytic oxidation
-
Paragraph 0026, (2021/08/06)
The invention provides a method for prep...
Bioisosteric investigation of ebselen: Synthesis and in vitro characterization of 1,2-benzisothiazol-3(2H)-one derivatives as potent New Delhi metallo-β-lactamase inhibitors
Jin, Wen Bin,Xu, Chen,Cheung, Qipeng,Gao, Wei,Zeng, Ping,Liu, Jun,Chan, Edward W.C.,Leung, Yun-Chung,Chan, Tak Hang,Wong, Kwok-Yin,Chen, Sheng,Chan, Kin-Fai
, (2020/04/30)
Carbapenem-resistant Enterobacteriaceae ...
2634-33-5 Process route
-
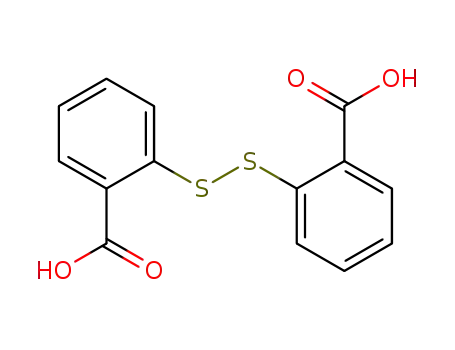
- 119-80-2
2,2'-dithiobenzoic acid

-
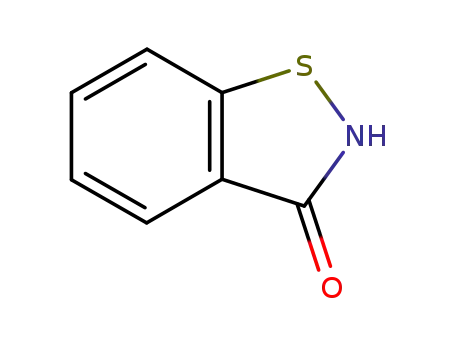
- 2634-33-5
1,2-benzisothiazolin-3-one
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1.1: aq. NaOH / 3 h / 25 °C
2.1: NaOMe; CH3CONH2 / toluene / 2 h / 80 °C
2.2: aq. H2O2 / 50 - 55 °C
2.3: 90 percent / aq. HCl / 25 °C
With acetamide; sodium hydroxide; sodium methylate; In toluene;
|
|
|
Multi-step reaction with 3 steps
1: 71 percent / SOCl2 / dimethylformamide; toluene / 18 h / 75 °C
2: Cl2 / CH2Cl2
3: concd NH4OH / CH2Cl2 / 1 h
With ammonium hydroxide; thionyl chloride; chlorine; In dichloromethane; N,N-dimethyl-formamide; toluene;
|
|
|
Multi-step reaction with 3 steps
1: thionyl chloride / Reflux
2: chlorine / tetrachloromethane / 20 °C
3: ammonia / dichloromethane / 2 h / -15 - 20 °C
With thionyl chloride; ammonia; chlorine; In tetrachloromethane; dichloromethane;
|
|
|
Multi-step reaction with 2 steps
1: thionyl chloride / Reflux
2: triethylamine; ammonia / dichloromethane / 12 h / 20 °C / Cooling with ice
With thionyl chloride; ammonia; triethylamine; In dichloromethane;
|
|
|
Multi-step reaction with 3 steps
1: oxalyl dichloride; N,N-dimethyl-formamide / dichloromethane / 3 h / 20 °C
2: ammonia / dichloromethane / 0 - 20 °C
3: bromine / dichloromethane / 0 - 20 °C
With oxalyl dichloride; ammonia; bromine; N,N-dimethyl-formamide; In dichloromethane;
|
|
|
Multi-step reaction with 2 steps
1: sulfur; chlorine / 1,1,2,2-tetrachloroethylene / 70 °C
2: ammonia / 1,2-dichloro-ethane / 3 h / 60 °C
With ammonia; chlorine; sulfur; In 1,1,2,2-tetrachloroethylene; 1,2-dichloro-ethane;
|
-
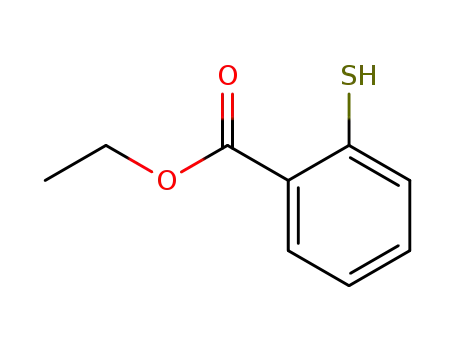
- 33441-56-4
ethyl 2-mercaptobenzoate

-

- 2634-33-5
1,2-benzisothiazolin-3-one
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: 98 percent / hydroxylamine-O-sulfonic acid (HOSA); KOH / H2O / 0.5 h / ice bath
2: 69 percent / KOH / ethanol / 0.5 h / 20 °C
With potassium hydroxide; hydroxylamine-O-sulfonic acid; In ethanol; water; 1: amination / 2: Cyclization;
|
2634-33-5 Upstream products
-
19602-82-5
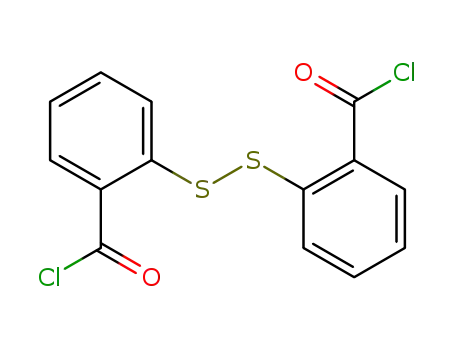
2,2'-dithiodibenzoic acid dichloride
-
1677-27-6
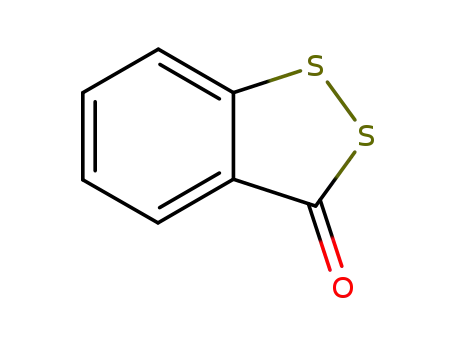
3H-1,2-benzodithiol-3-one
-
2527-57-3
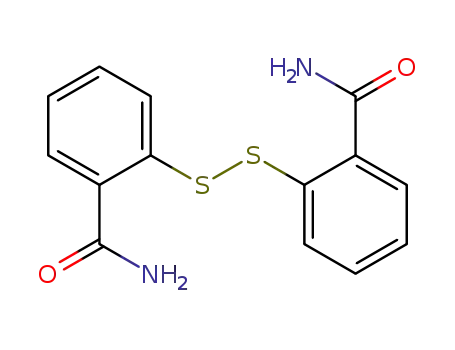
2,2'-dithiobisbenzamide
-
34263-64-4
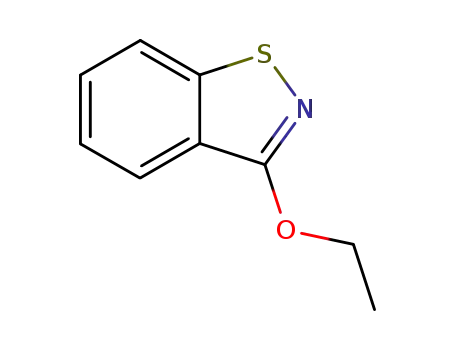
3-ethoxy-1,2-benzisothiazole
2634-33-5 Downstream products
-
17927-88-7
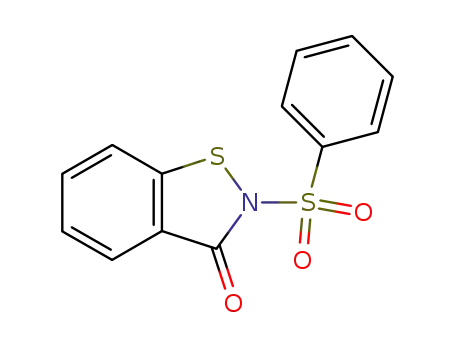
2-(phenylsulfonyl)benzo[d]isothiazol-3(2H)-one
-
101793-97-9
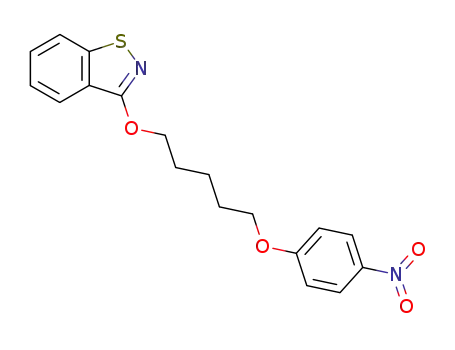
3-[5-(4-nitro-phenoxy)-pentyloxy]-benz[d]isothiazole
-
100-47-0

benzonitrile
-
7716-66-7
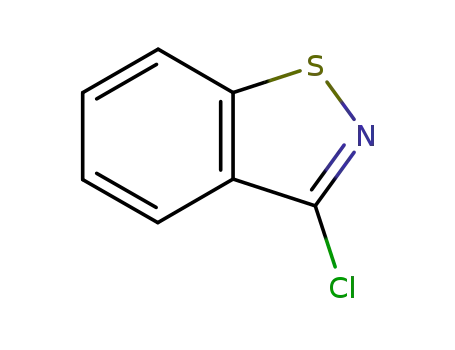
3-chloro-1,2-benzisothiazole
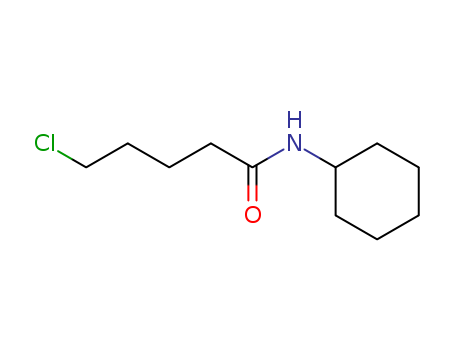
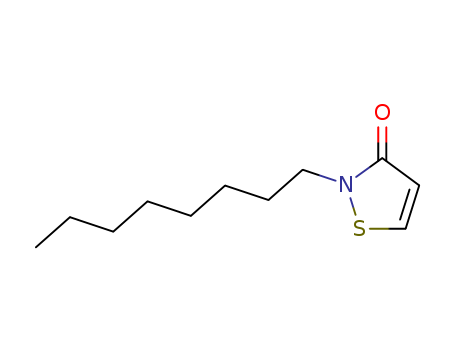
Relevant Products
-
N-Cyclohexyl-5-Chloropentanamide
CAS:15865-18-6
-
2-Octyl-4-Isothiazolin-3-One
CAS:26530-20-1