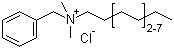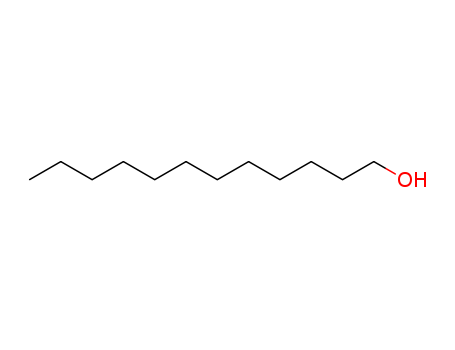
112-53-8
- Product Name:C12-14 Alcohol
- Molecular Formula:C12H26O
- Purity:99%
- Molecular Weight:186.338
Product Details;
CasNo: 112-53-8
Molecular Formula: C12H26O
Appearance: white low melting crystalline solid
Chinese Manufacturer Supply C12-14 Alcohol 112-53-8 On Stock with Competitive Price
- Molecular Formula:C12H26O
- Molecular Weight:186.338
- Appearance/Colour:white low melting crystalline solid
- Vapor Pressure:0.1 mm Hg ( 20 °C)
- Melting Point:22-27 °C
- Refractive Index:n20/D 1.442(lit.)
- Boiling Point:258 °C at 760 mmHg
- PKA:15.20±0.10(Predicted)
- Flash Point:115.4 °C
- PSA:20.23000
- Density:0.831 g/cm3
- LogP:3.89960
1-Dodecanol(Cas 112-53-8) Usage
|
Physical properties |
1-Dodecanol is a white low melting crystalline solid that has a melting point of 24°C.The air odor threshold for dodecyl alcohol (isomer not specified) is reported to be 7.1 ppb. |
|
Definition |
ChEBI: 1-Dodecanol is a fatty alcohol that is dodecane in which a hydrogen from one of the methyl groups is replaced by a hydroxy group. It is registered for use in apple and pear orchards as a Lepidopteran pheromone/sex attractant, used to disrupt the mating behaviour of certa n moths whose larvae destroy crops. |
|
Preparation |
Commercially 1-Dodecanol may be prepared by hydrogenation of lauric acid; normally employed as a replacement for the corresponding aldehyde. |
|
Production Methods |
1-Dodecanol is produced commercially by the oxo process and from ethylene by the Ziegler process, which involves oxidation of trialkylaluminum compounds. It can also be produced by sodium reduction or high-pressure hydrogenation of esters of naturally occurring lauric acid. |
|
Aroma threshold values |
Detection: 73 to 820 ppb |
|
Synthesis Reference(s) |
Journal of the American Chemical Society, 108, p. 6036, 1986 DOI: 10.1021/ja00279a061Tetrahedron Letters, 37, p. 3623, 1996 DOI: 10.1016/0040-4039(96)00652-1 |
|
General Description |
Colorless thick liquid with a sweet odor. Floats on water. Freezing point is 75°F. |
|
Reactivity Profile |
Dodecyl alcohol is an alcohol. Flammable and/or toxic gases are generated by the combination of alcohols with alkali metals, nitrides, and strong reducing agents. They react with oxoacids and carboxylic acids to form esters plus water. Oxidizing agents convert them to aldehydes or ketones. Alcohols exhibit both weak acid and weak base behavior. They may initiate the polymerization of isocyanates and epoxides. |
|
Health Hazard |
Liquid will cause burning of the eyes and may irritate skin. |
|
Flammability and Explosibility |
Nonflammable |
|
Safety Profile |
Moderately toxic by intraperitoneal route. Mildly toxic by ingestion. A severe human skin irritant. Questionable carcinogen with experimental tumorigenic data. Combustible when exposed to heat or flame; can react with oxidizing materials. To fight fire, use dry chemical, CO2. When heated to decomposition it emits acrid smoke and irritating fumes |
|
Carcinogenicity |
1-Dodecanol showed weak tumor-promoting activity when applied three times a week for 60 weeks to the skin of mice that had previously received an initiating dose of dimethylbenz[a]anthracene. Papillomas developed in 2 of 30 mice after 39 and 49 weeks of treatment. |
|
Purification Methods |
Crystallise dodecanol from aqueous EtOH, and distil it through a spinning-band column under vacuum. [Ford & Marvel Org Synth 10 62 1930, Beilstein 1 IV 1844.] |
InChI:InChI=1/C12H26O/c1-2-3-4-5-6-7-8-9-10-11-12-13/h13H,2-12H2,1H3
112-53-8 Relevant articles
Kinetics of the Acid-catalysed Hydrolysis of Dodecylsulphate and Dodecyldiethoxysulphate Surfactants in Concentrated Micellar Solutions. Part 1. - Effects of Acid and Surfactant Concentrations and of the Nature and Concentration of Counterions
Garnett, Christopher J.,Lambie, Alan J.,Beck, William H.,Liler, Milica
, p. 953 - 964 (1983)
The rates of the acid-catalysed hydrolys...
-
Church,Abdel-Gelil
, p. 813,816 (1957)
-
Acid-catalyzed hydrolysis of sodium dodecyl sulfate
Nakagaki,Yokoyama
, p. 1047 - 1052 (1985)
The acid-catalyzed hydrolysis of sodium ...
The hydrolysis of C12 primary alkyl sulfates in concentrated aqueous solutions. Part 1. General features, kinetic form and mode of catalysis in sodium dodecyl sulfate hydrolysis
Bethell,Fessey,Namwindwa,Roberts
, p. 1489 - 1495 (2001)
As part of an investigation into the obs...
An effective hydrolysis of crowded chiral esters
Vávra, Jan,Streinz, Ludvík,Vodi?ka, Petr,Budě?ínsky, Milo?,Koutek, Bohumír
, p. 1886 - 1888 (2002)
Trifluoromethanesulfonic acid-coated sil...
A practical and efficient procedure for reduction of carboxylic acids and their derivatives: use of KBH4-MgCl2
Qiu, You-Chun,Zhang, Fu-Li,Zhang, Chun-Nian
, p. 7595 - 7598 (2007)
The use of KBH4-MgCl2 to reduce carboxyl...
Cleavage of protecting groups catalysed by π-acceptors
Tanemura, Kiyoshi,Nishida, Yoko,Suzuki, Tsuneo,Satsumabayashi, Koko,Horaguchi, Takaaki
, p. 40 - 41 (1999)
The cleavage of protecting groups is cau...
-
Watanabe,A.
, p. 398 - 402 (1961)
-
Selective conversion of coconut oil to fatty alcohols in methanol over a hydrothermally prepared Cu/SiO2 catalyst without extraneous hydrogen
Wu, Liubi,Li, Lulu,Li, Bolong,Zhao, Chen
, p. 6152 - 6155 (2017)
A novel one-pot approach selects a hydro...
Thermodynamics of the lipase-catalyzed esterification of 1-dodecanoic acid and 1-dodecanol in organic solvents
Tewari
, p. 750 - 755 (1998)
Lipase immobilized on controlled-pore gl...
A glucose-activatable trimodal glucometer self-assembled from glucose oxidase and MnO2 nanosheets for diabetes monitoring
Chen, Jin-Long,Li, Li,Wang, Shuo,Sun, Xiao-Yan,Xiao, Lu,Ren, Jia-Shu,Di, Bin,Gu, Ning
, p. 5336 - 5344 (2017)
Daily monitoring of blood glucose is of ...
NEW GLYCOSPHINGOLIPIDS FROM MARINE ORGANISM
Hirsch, S.,Kashman, Y.
, p. 3897 - 3906 (1989)
The structure of several ceramides and c...
Benzimidazoline-dimethoxypyrene. An effective promoter system for photoinduced electron transfer promoted reductive transformations of organic compounds
Hasegawa, Eietsu,Hirose, Harumi,Sasaki, Kosuke,Takizawa, Shinya,Seida, Takayuki,Chiba, Naoki
, p. 1147 - 1161 (2009)
2-(p-Methoxyphenyl)-1,3-dimethylbenzimid...
Reaction Pathway and Selective Hydrogenation on Catalyst Derived from Oxidation Treatment of Mg2Cu Alloy
Chikamatsu, Nobuyasu,Tagawa, Tomohiko,Goto, Shigeo
, p. 1548 - 1552 (1994)
A catalyst derived from oxidation treatm...
Transformation of methyl laurate into lauryl alcohol over a Ru-Sn-Mo/C catalyst by using zerovalent iron and water as an in situ hydrogen source
Sagata, Kunimasa,Hirose, Mina,Hirano, Yoshiaki,Kita, Yuichi
, p. 85 - 91 (2016)
Hydrogenation and hydrogenolysis reactio...
Influence of Higher Alcohols on Acid-Catalyzed Hydrolysis of Sodium Dodecyl Sulfate. Effect of Complex Formation
Nakagaki, Masayuki,Yokoyama, Shoko
, p. 935 - 936 (1986)
The order of effectiveness of 1-alkanols...
Structural characterization of solid trivalent metal dodecyl sulfates: From aqueous solution to lamellar superstructures
Pereira, Rui F. P.,Valente, Artur J. M.,Burrows, Hugh D.,De Zea Bermudez, Veronica,Carvalho, Rui A.,Castro, Ricardo A. E.
, p. 1420 - 1433 (2013)
Metal dodecyl sulfates of trivalent alum...
Preparation of cyclohexene-d10 by H/D-exchange reaction
Ishibashi, Kenichi,Matsubara, Seijiro
, p. 724 - 725 (2007)
Preparation of fully deuterium-labeled c...
Anchored Aluminum Catalyzed Meerwein-Ponndorf-Verley Reduction at the Metal Nodes of Robust MOFs
Larson, Patrick J.,Cheney, Joseph L.,French, Amanda D.,Klein, David M.,Wylie, Benjamin J.,Cozzolino, Anthony F.
, p. 6825 - 6832 (2018)
Catalytic Meerwein-Ponndorf-Verley reduc...
Lithium amidotrihydroborate, a powerful new reductant. Transformation of tertiary amides to primary alcohols
Myers, Andrew G.,Yang, Bryant H.,Kopecky, David J.
, p. 3623 - 3626 (1996)
Lithium amidotrihydroborate (LiH2NBH3, L...
Mechanism of supported Ru3Sn7 nanocluster-catalyzed selective hydrogenation of coconut oil to fatty alcohols
Luo, Zhicheng,Bing, Qiming,Kong, Jiechen,Liu, Jing-Yao,Zhao, Chen
, p. 1322 - 1332 (2018)
As a promising hydrotreating catalyst, i...
Elucidation of the mechanism of titanocene-mediated epoxide opening by a combined experimental and theoretical approach
Daasbjerg, Kim,Svith, Heidi,Grimme, Stefan,Gerenkamp, Mareike,Mueck-Lichtenfeld, Christian,Gansaeuer, Andreas,Barchuk, Andriy,Keller, Florian
, p. 2041 - 2044 (2006)
(Figure Presented) Steric effects govern...
Highly efficient conversion of fatty acids into fatty alcohols with a Zn over Ni catalyst in water
Gao, Xia,Tong, Daming,Zhong, Heng,Jin, Binbin,Jin, Fangming,Zhang, Hua
, p. 27623 - 27626 (2016)
A new route to convert fatty acids into ...
Deactivation mechanism of Cu/Zn catalyst poisoned by organic chlorides in hydrogenation of fatty methyl ester to fatty alcohol
Huang, Hui,Wang, Shaohong,Wang, Shujia,Cao, Guiping
, p. 351 - 357 (2010)
The mechanism of deactivation of Cu/Zn c...
Water: The ideal hydrogen-atom source in free-radical chemistry mediated by TiIII and other single-electron-transfer metals?
Cuerva, Juan M.,Campa?a, Araceli G.,Justicia, José,Rosales, Antonio,Oller-López, Juan L.,Robles, Rafael,Cárdenas, Diego J.,Bu?uel, Elena,Oltra, J. Enrique
, p. 5522 - 5526 (2006)
(Chemical Equation Presented) Hydrogen-a...
Manganese-Catalyzed Hydrogenation of Sclareolide to Ambradiol
Zubar, Viktoriia,Lichtenberger, Niels,Schelwies, Mathias,Oeser, Thomas,Hashmi, A. Stephen K.,Schaub, Thomas
, (2021/11/16)
The hydrogenation of (+)-Sclareolide to ...
Hydrodeoxygenation of non-edible bio-lipids to renewable hydrocarbons over mesoporous SiO2-TiO2 supported NiMo bimetallic catalyst
Ba, Wenxia,Fu, Lin,Li, Xin,Li, Yongfei,Liu, Yuejin,Zhang, Simiao,Zhao, Jingxuan
, (2022/02/17)
Ni-catalysts are promising candidate for...
Defunctionalization of sp3 C–Heteroatom and sp3 C–C Bonds Enabled by Photoexcited Triplet Ketone Catalysts
An, Juzeng,Gu, Yiting,Martin, Ruben,Wakeling, Matthew,Yin, Hongfei
, p. 1031 - 1036 (2022/01/19)
A general strategy for enabling a light-...
Enantiomeric synthesis of natural alkylglycerols and their antibacterial and antibiofilm activities
Fernández Montoya, Deicy J.,Contreras Jordan, Luis A.,Moreno-Murillo, Bárbara,Silva-Gómez, Edelberto,Mayorga-Wandurraga, Humberto
supporting information, p. 2544 - 2550 (2019/11/13)
Alkylglycerols (AKGs) are bioactive natu...
112-53-8 Process route
-
- 112-66-3
lauryl acetate

-
- 70770-06-8
1-benzyloxy-3-phenylpropane

-
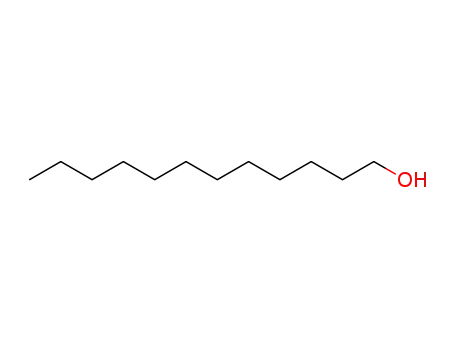
- 112-53-8
1-dodecyl alcohol

-
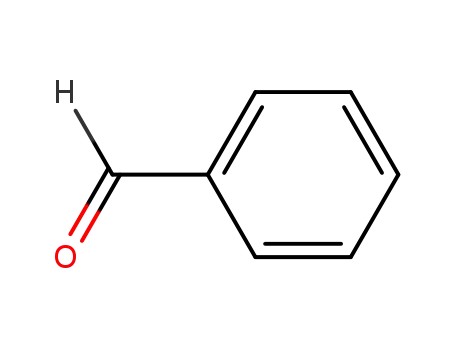
- 100-52-7
benzaldehyde

-
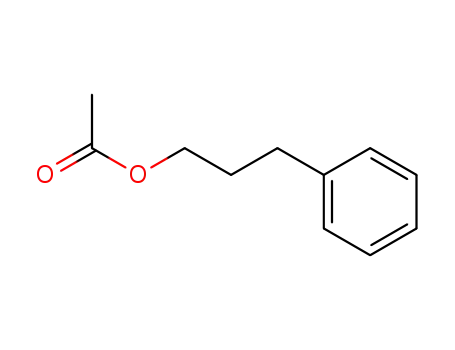
- 122-72-5
3-phenylpropyl acetate
| Conditions | Yield |
|---|---|
|
With Bromotrichloromethane; (4,4'-di-tert-butyl-2,2'-dipyridyl)-bis-(2-phenylpyridine(-1H))-iridium(III) hexafluorophosphate; In dichloromethane; at 20 ℃; for 12h; Inert atmosphere; Irradiation;
|
-
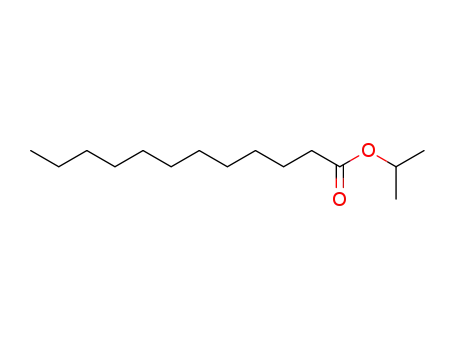
- 10233-13-3
lauric acid isopropyl ester

-

- 112-53-8
1-dodecyl alcohol

-

- 2437-25-4
undecyl cyanide
| Conditions | Yield |
|---|---|
|
lauric acid isopropyl ester; With diisobutylaluminium hydride; In hexane; dichloromethane; at -78 ℃; for 3h; Inert atmosphere;
With ammonia; iodine; In tetrahydrofuran; hexane; dichloromethane; water; at -78 - 20 ℃; Inert atmosphere;
|
78% 6% |
112-53-8 Upstream products
-
143-07-7
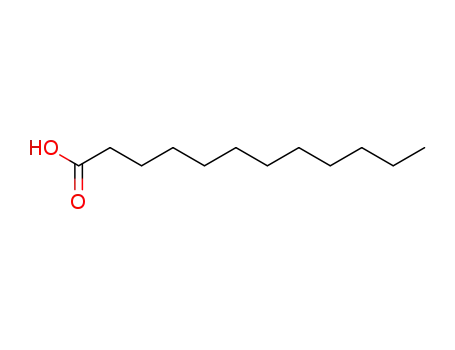
lauric acid
-
112-16-3
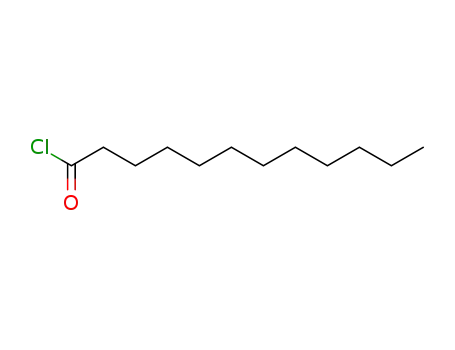
n-dodecanoyl chloride
-
60-29-7
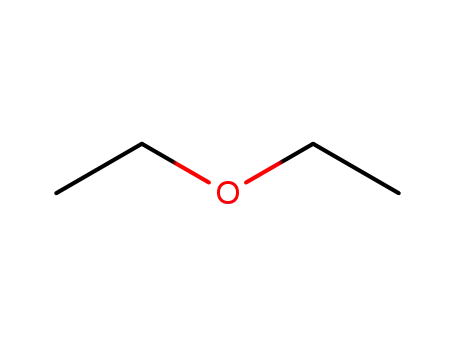
diethyl ether
-
677-22-5
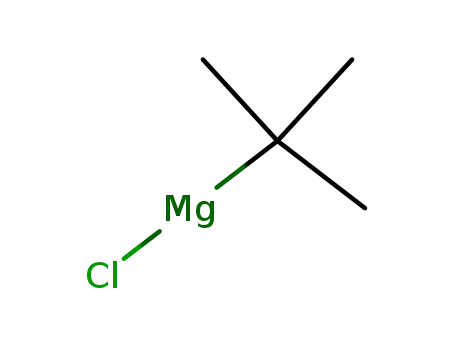
tert-butylmagnesium chloride
112-53-8 Downstream products
-
48073-44-5
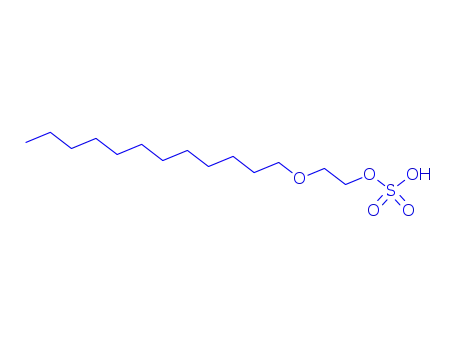
Dodecyloxyaethylenschwefelsaeure
-
4536-30-5
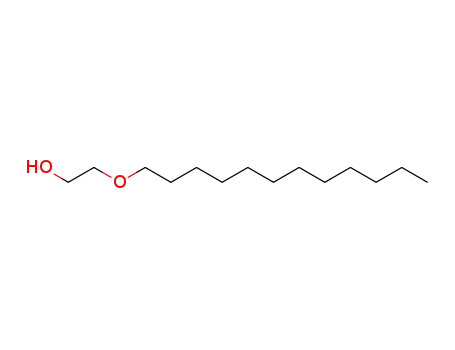
ethylene glycol mono-n-dodecyl ether
-
5917-47-5

1-dodecylpiperidine
-
13945-76-1
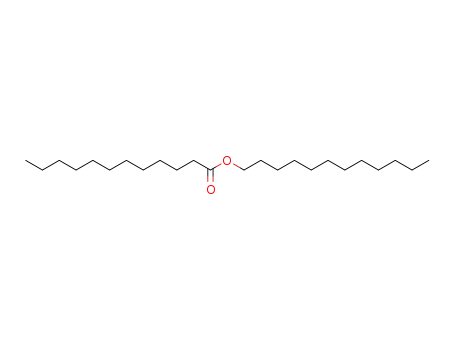
dodecyl laurate
Relevant Products
-
5-(2′-Hydroxy-3′-Naphthamide)-2-Benzimidazolone
CAS:26848-40-8
-
Benzalkonium Chloride
CAS:63449-41-2
-
Didecyl Dimethyl Ammonium Chloride
CAS:7173-51-5